| Pages:
1
2
3 |
cyanureeves
National Hazard
   
Posts: 744
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
also i know my question is not about distillation but it is about DYI. i rigged up a stir em up contraption with a fan and a big ole magnet and it
spins a screw pretty good in a water filled jar. i got it set on a wooden box i made and have to toy with the height or it will spin a vortex until it
makes a toilet flushing sound and acts all stupid. now i need a stir bar so i got a headless screw suspended in some silicone rtv and i'm thinking of
shaving it into shape tommorrow or whenever it cures.i dont know if silicone would be right for an acetone and hypochlorite mixture as in
chloroform,or even hydrazine synthesis.last time i made chloroform i swirled it around myself in freezing weather and that sucked!what can serve as a
stir bar for now until i get some money to buy one? thank you very much.
|
|
|
Arthur Dent
National Hazard
   
Posts: 553
Registered: 22-10-2010
Member Is Offline
Mood: entropic
|
|
I completely support home chemistry and I commend people who create cool DIY apparatus and home-brewed gear, but I think there are limits to
McGuyver-isms...
A stirbar can be had for as little as $2 on Ebay, and most hobby shops and online science/hobby stores have these for even less! Surely you can spare
a few bucks for proper gear?
Your idea, however commendable, has a few flaws. Even cured, the silicone rubber will not glide and slide properly at the bottom of your flask/jar. So
it will probably bump and rattle out of control, not really mixing anything. Plus most mason jars and condiment jars have a bottom surface that is not
flat, but slightly domed upwards... which again is not a friendly environment for a stirbar. I strongly suggest that you use a jar with a flat or
recessed bottom, and that you do not use a screw dipped in goop!
A suggestion for an alternative stirbar is to use a bit of glass tubing about an inch long, find a nail that fits the bore snugly, clip both ends of
the nail to a bit over 1/2" and then heat seal both ends with a torch... Voilà! A stirbar that is impervious to any chemical attack, but be careful
when you "drop" it in a jar as it will be quite fragile.
Robert
--- Art is making something out of nothing and selling it. - Frank Zappa ---
|
|
|
food
Hazard to Self
 
Posts: 86
Registered: 4-9-2010
Location: the West
Member Is Offline
Mood: mithering
|
|
Something that I've been doing is to mate my bona fide glass condenser to larger glass jugs with sections cut from bicycle inner tubes. Depending on
the diameter of the jug mouth the length of tube can be a simple ring or a longer piece doubled back on itself. Typically I'll do this with a 160 fl
oz glass jug.
I've not done this with anything involving particularly aggressive chemicals or temperatures. It's just occurred to me that the rubber could be
isolated with teflon tape.
The base of the jugs that I have aren't flat, but stirring with a magnetic stir bar has been working.
I'd thought about adding that to the thread about lab tips. Inner tubes are a great resource, and are there for the asking. Only a couple of weeks ago
I saw a shop display of locally made accessories (for the fairer sex) manufactured from inner tube material. A kind of gritty gothish look.
|
|
|
cyanureeves
National Hazard
   
Posts: 744
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
if you're going to use rubber then that so called space age rubber looking material used for cooking should work better. it's starting to show up at
resale shops and flea markets more and more because older people don't like it. i got a couple strown around in my tool shed.
|
|
|
food
Hazard to Self
 
Posts: 86
Registered: 4-9-2010
Location: the West
Member Is Offline
Mood: mithering
|
|
not so much a goal, but it's been a handy setup for volumes too large for the flasks that I have
Quote: Originally posted by cyanureeves  |
then that so called space age rubber looking material used for cooking should work better. it's starting to show up at resale shops and flea markets
more and more because older people don't like it. i got a couple strown around in my tool shed. |
Isn't that material silicone? Some possibilities there; a section of a glove finger might do the same thing as my inner tube piece.
I may be one of those older people. It did strike me as an odd material to be involved in the cooking or baking process. I haven't tried it yet.
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
This perhaps one of the better "home manufactured still" design manuals and
goes into detail quite explicitly.
Revamping this to handle corrosive materials would be very simple (a switch in materials, etc) and offers a very expedient method of working with
non-corrosives, as is.
Attachment: Ethanol_still_design.pdf (1.5MB)
This file has been downloaded 1240 times
|
|
|
food
Hazard to Self
 
Posts: 86
Registered: 4-9-2010
Location: the West
Member Is Offline
Mood: mithering
|
|
Quote: Originally posted by quicksilver  | This perhaps one of the better "home manufactured still" design manuals and
goes into detail quite explicitly.
Revamping this to handle corrosive materials would be very simple (a switch in materials, etc) and offers a very expedient method of working with
non-corrosives, as is. |
it's funny that you should post that. I made a still from this design, with some minor modifications.
I ran it this time with an oven thermometer probe through to the side-arm takeoff. Before now I've used a glass thermometer, but it's mercury, and
I've been getting nervous about mishaps. Works like a hot damn. Used it for ethanol so far. I have a proof hydrometer and iirc it's around 95%.
The stainless steel stock pot lid has a copper union(?) attached. The column drops into that; not permanently attached. The weight of the column
pretty much seals the lid of the stock pot. I've done different things for that. Typically draping heavier teflon tape around the edge. I think that
the last time I just put the lid on and went naked.
Made the feed material from a crude (and cheap!) sugar sold in east indian stores. Comes in a large block. My thinking is that we're looking at a
material that is rich in nutrients. No need to fret over special 'turbo' yeast products. It's very crude; bugs, everything; jaggary. Lavalin EC-1118
provides a moderately alcohol tolerant yeast for higher initial alcohol. Last time I froze a huge mother pail of the fermented stuff to achieve an
even higher alcohol %, and lower volume.
sorry about the mess (standard disclaimer, girls)

|
|
|
Arthur Dent
National Hazard
   
Posts: 553
Registered: 22-10-2010
Member Is Offline
Mood: entropic
|
|
@ food: Nice work mate! Been thinking about doing something of that sort, but for now, my strong spirits still consists of two boiling flasks, a
claisen adapter with thermometer and a pyrex graham condenser. I'm strictly small production, and the only time I pull out the big 5 gallon glass
bottle is when I brew beer.
I have part of my glassware collection that's dedicated only to make ethanol for consumption. It's either been bought new or has been treated
thoroughly with acids and cleaned extensively. I don't use that glassware when I work with heavy metals like manganese, nickel and copper.
Robert
--- Art is making something out of nothing and selling it. - Frank Zappa ---
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
It (the design) does indeed work. I have used a very nice 2 liter kit that came in a briefcase for some years but needed to make some chloroform and
other odd and ends so I tried out the design and liked it too.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
@food
That is a really nice ethanol still. I don't think anything like that is used for distilling HNO3 normally though. A column could be used industrially
to bring dilute HNO3 up to the azeotrope (~68wt% HNO3) I guess before using dehydrating agents to bring the concentration up past the azeotrope.
I think for the hobbyist a nitrate salt, or sometimes dilute HNO3, is normally added to H2SO4 and distilled to give concentrated or greater than
concentrated HNO3 directly using a pot still basically (no column).
Just for fun last night I played around with an improvised set up for distilling HNO3. The basic design is nothing new; I have seen similar things
done in several different places. I used 40g of AN and 25mL 90% H2SO4. I collected ~23mL of ~60wt% HNO3 (wt% determined from density and tables). I
didn't get a great yield, but in an hour with this very simple set up it is possible to get a little acid to play with. Except for the beaker all the
other components were just things that were lying around (household items, not lab items).
 
BTW, the stem on the wine glass thing I am using for my distillate receiver is really too thick. A thinner stem would have greater thermal resistance,
which would result in less heat transfer between the lower boiling area and the receiver cup.
Some of the ideas above for an improvised Liebig condenser would make for a much more productive set up. As suggested glass tubing could be run
through a plastic water jacket to make the condenser. Any glass flask that will handle being heated could be used for the boiling vessel. A nice
teflon stopper could be turned on a lathe (find a piece of teflon and get someone with a lathe to turn it into a stopper shape. The stopper and the
hole in the center could be sized in such a way that teflon tape could be wrapped around the glass tubing to make it fit the stopper hole and around
the outside of the stopper to make it fit the flask (boiling vessel). This setup should work well, I think.
[Edited on 15-10-2011 by Hennig Brand]
|
|
|
unsub
Harmless

Posts: 5
Registered: 23-10-2011
Member Is Offline
Mood: No Mood
|
|
Hello all this is my first post. I have a couple old style flasks etc and was considering buying the condensor to go with them but will probably just
splurge for ground glass so the comment about 19/22 is interesting. I like the idea of "juryrigging" a kit together though and like the PVC condenser
which I suppose could be quite long if need be. My idea for home built 3 neck reaction flask would have pyrex casserole dish bottom ,teflon tape
gasket with a flat piece of stainless steel with your 3 holes "C" clamped together. The steel could be painted with teflon paint and you could even
fit the holes with stainless pipe carefully dremelled to 19/22 or 24/40 and use it with actual ground glass. I would want room to wrap the glass in
teflon tape though. I don't want to spend a week of evenings on the steel lid if there is an obvious flaw I have missed?
The question of hooking up the glass tube of the PVC condenser would be simple if not to corrosive since you could just use rubber stoppers and
silicon tubing. For corrosive chemicals on a budget I would steal an idea from vogels and use a cork stoppr wrapped in aluminum foil(very carefully)
to connect the glass tube from the condenser to the stainless and teflon reaction vessel.
Another idea I have been toying with is using a clear glass teapot as a makeshift buchner flask. Attach the vacuum hose to the spout.
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 860
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
Quote: Originally posted by unsub  | | The steel could be painted with teflon paint and you could even fit the holes with stainless pipe carefully dremelled to 19/22 or 24/40 and use it
with actual ground glass. |
There's no way you'll get a remotely good seal without using a precision lathe.
I don't get it why some people actually are willing to pour hours of thinking and MacGyvering into projects like these when a three-neck RBF can be
had from eBay at quite reasonable prices (and you're sure it will work).
Welcome, by the way.
This just in: 95,5 % of the world population lives outside the USA
Please drop by our IRC channel: #sciencemadness @ irc.efnet.org
|
|
|
unsub
Harmless

Posts: 5
Registered: 23-10-2011
Member Is Offline
Mood: No Mood
|
|
Thank you lambada-eyde for the welcome.
I enjoy "macguvering" as an end in it's self and totally agree that simply ordering a proper flask is much much more efficient use of time. It is
also a safety hazard with some chemicals.
However depending where you liveordering glassware and chemicals invites a lot of scrutiny from the secret police. I like to think that making a
distillation kit from everyday items would prove to the powers that be that there is no point in banning or restricting laboratory glassware.
I like coffeemaker pots for beakers since they can handle heat. Mine even has marked graduations although I should write out a metric to "cups of
coffee" scale.
I disagree about not getting a good seal though. I make knives as a hobby and can fit pieces together so a layer or 2 of teflon tape can easily seal
it. If I can make something out of mostly recycled consumer goods that is worth some extra effort and if some politician says we shouldn't have one
then it's a matter of principle.
|
|
|
zgoat65
Harmless

Posts: 11
Registered: 27-1-2012
Member Is Offline
Mood: No Mood
|
|
If someone lives in the Lone Star State, one 5000ml three-neck flask with no chemical present for a mile can aquire someone an all expense paid
vacation (for ten years ) at one of the lovely state funded resorts. Just the glass can get you a case.
[Edited on 28-1-2012 by zgoat65]
zgoat65
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Quote: Originally posted by zgoat65  | If someone lives in the Lone Star State, one 5000ml three-neck flask with no chemical present for a mile can aquire someone an all expense paid
vacation (for ten years ) at one of the lovely state funded resorts. Just the glass can get you a case.
[Edited on 28-1-2012 by zgoat65] |
Can you give an example of that law being used to convict someone who
wasn't actually making illegal drugs? I don't think it has been done.
|
|
|
zgoat65
Harmless

Posts: 11
Registered: 27-1-2012
Member Is Offline
Mood: No Mood
|
|
[Edited on 28-1-2012 by zgoat65][/rquote]Can you give an example of that law being used to convict someone who wasn't actually making illegal
drugs? I don't think it has been done.[/rquote]
Here is a useless link that gives some opinion and truth on the law in my fair state. http://thegoodreverend.blogspot.com/2006/04/texass-anti-lab-... .
While I have found nothing on a conviction of anyone
possessing lab glass alone, most had p2p or multiplereagents in town with the glass. I will say that they most undoubtedly charge uou with a crime,
and possibly with intent to manufacture. I was charged with possession of chemicals with intent for having camp fuel, drain cleaner (sulfuric), and
brake fluid (never understood this) in a vehicle I was a passenger in (and all but one chem in the trunk _ie drain cleaner) with an explanation for
all itens, they still charged the drivera and myself with a crime and had to post a 10000 dollar bond. While the case was thrown out due to it. being
stupid I still had to fork out 1000 dollars to maintain my freedom, as well as having this arrests show up on any future background check. CAN they
convicted on on basic possession of lab glass? I hace no clue. Will they try? The answers is YES!
zgoat65
|
|
|
zgoat65
Harmless

Posts: 11
Registered: 27-1-2012
Member Is Offline
Mood: No Mood
|
|
[Edited on 28-1-2012 by zgoat65][/rquote]Can you give an example of that law being used to convict someone who wasn't actually making illegal
drugs? I don't think it has been done.[/rquote]
Here is a useless link that gives some opinion and truth on the law in my fair state. http://thegoodreverend.blogspot.com/2006/04/texass-anti-lab-... .
While I have found nothing on a conviction of anyone
possessing lab glass alone, most had p2p or multiplereagents in town with the glass. I will say that they most undoubtedly charge uou with a crime,
and possibly with intent to manufacture. I was charged with possession of chemicals with intent for having camp fuel, drain cleaner (sulfuric), and
brake fluid (never understood this) in a vehicle I was a passenger in (and all but one chem in the trunk _ie drain cleaner) with an explanation for
all itens, they still charged the drivera and myself with a crime and had to post a 10000 dollar bond. While the case was thrown out due to it. being
stupid I still had to fork out 1000 dollars to maintain my freedom, as well as having this arrests show up on any future background check. CAN they
convicted on on basic possession of lab glass? I hace no clue. Will they try? The answers is YES!
zgoat65
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I forgot about this thread and made a post about a simple homemade glass distillation apparatus made from common flasks and materials in a nitric acid
synthesis thread. It fits in that thread too though I guess.
http://www.sciencemadness.org/talk/viewthread.php?tid=2176&a...
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I made a few changes to the simple still I posted pictures and a description of in the nitric acid synthesis thread. A thermometer port (hole) was
added (drilled) into the Teflon bushing on the Erlenmeyer boiling flask. A bushing was also added to the receiving flask as well as a small
centrifugal pump so that a constant supply of cool water could be made to flow over the round bottom receiver. A small diameter glass tube was used to
vent the system and prevent buildup of pressure or vacuum. The vent tube's open end was kept well away from the water bath and flowing water so as to
reduce absorption of moisture by the produced nitric acid.
It works extremely well. The thermometer in particular is a very valuable addition. Depending on the way you look at it, the old quantity versus
purity trade off, I botched the distillation. To produce the HNO3, 480g of NH4NO3 and 300mL of 91%
H2SO4 were used. I ended up with a rather large volume of distillate (234mL), but it titrated at only 82wt% HNO3. For
the last 45 minutes or so of distillation the temperature of the vapor sat right at about 120C. When it hit 120C is when I probably should have made
the cut. Oh well, I have more acid now, but it does need to be redistilled with concentrated sulfuric acid if I want over 95wt% acid. The sulfuric
acid was drain cleaner that titrated at only 91% and the ammonium nitrate used was fertilizer prills and looked and felt a bit damp (should have been
dried).
A significant amount of cooling can be had through evaporative cooling, with this arrangement, if the cooling water only needs to be kept slightly
above the wet bulb temperature of the ambient air.
The bushing on the receiving flask is not Teflon, but is a bushing I made from a random piece of plastic round stock found at a scrap yard before I
got a piece of Teflon. It is very resistant to nitric acid, only showing very slight blistering when used on the nitric acid boiling flask for several
hours. I used it on the boiling flask a year ago or so. I tried yet another piece of plastic round stock from the same scrap yard and it turned to
bubbly, tar, goop in about ten minutes on exposure to hot nitric acid. I don't know what either of the plastics are, but it is easy to tell that they
are different from Teflon and each other by the way they cut and drill, etc, on the lathe.
Showing off my recently made homemade electric kiln in the pictures. 
 
 
Just for the hell of it 4mL of the newly distilled nitric acid was mixed with 8mL of 91% sulfuric acid and used to nitrate 2mL of glycerine. The
glycerine was added to the mixed acid in a 50mL beaker drop wise from a pipette with swirling and then covered in plastic wrap and left for an hour
and a half at room temperature out of the sun. The yield was surprisingly about 2mL of NG from 2mL of glycerine. A lot of the acids I have used in the
past were probably not nearly as concentrated as I thought they were.
[Edited on 16-7-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Sorry for the multiple posting, but I have a small correction for the above post. The receiving flask used was actually a Florence flask, not a round
bottom flask as was previously stated. The longer neck of a Florence flask may actually be better in this case as compared to the normally shorter
neck of a round bottom flask. I guess some round bottom flasks do have long necks as well though.
Yeah, the end of that vent tube probably shouldn't end where it does either. I was at a different location entirely when I cut the piece of tubing and
guesstimated the length.
[Edited on 20-7-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Found a really good webpage with information regarding recovery, concentration and purification of mineral acids. I attached an image of the nitric
acid graphs, which I thought would be very useful for someone synthesizing or concentrating nitric acid (distilling).
http://www.ddpsinc.com/de-dietrich-services
Was having a little trouble linking to the exact page; the link above is a couple clicks away from the page on mineral acids. I made a pdf of the
mineral acids page and attached it below.
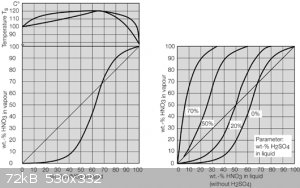
Attachment: Recovery, Concentration and Purification of Mineral Acids DDPS.pdf (341kB)
This file has been downloaded 894 times
[Edited on 26-8-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
The following is the bit of text which preceded the graphs which where attached in the last post.
"The diagrams below (show) the impact of different sulfuric acid concentrations to the azeotrope point. Azeotrope disappears beyond a sulfuric acid
concentration of 50 wt%, yet for technical rectification, an acid concentration of about 70 wt% is to be targeted."
I made an Excel spreadsheet this morning which I think will be a decent tool to use when concentrating nitric acid using sulfuric acid to break the
HNO3/H2O azeotrope. To simplify, all water was assumed to remain in the boiler; when this becomes significantly inaccurate can easily be determined by
referring to the vapor-liquid equilibrium diagram included with the spreadsheet.
Attachment: Concentrating HNO3 - Overcoming the HNO3_H2O Azeotrope Using H2SO4.xlsx (79kB)
This file has been downloaded 607 times
Attachment: Concentrating HNO3 - Overcoming the HNO3_H2O Azeotrope Using H2SO4 (compatibility mode).xls (97kB)
This file has been downloaded 643 times
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Quote: Originally posted by hissingnoise  | I just cannot understand why people continue to fuck around with stoppers, teflon tape, alufoil and that shit when a simple all-glass apparatus costs
a few euro . . .
|
Me too! I worked with a geophysicist that liked to make things .. as an example he bought $2 worth of mesh and spent an evening sewing a mosquito
hood. I bought one made well enough to exclude the little bastards for $4.50. These are apparatus hobbyists and people who devalue the worth of their
time. I was getting a day rate so time spent fucking around with gear was money stolen from the client.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Sounds like kind of an extreme example. 
Well, I think learning the simple skill of making Teflon bushings, allowing the hobbyist to make their own safe and reliable distillation and other
equipment while eliminating or significantly reducing the need for glassblowing, is very worthwhile. Plus if the situation ever arises, you will have
developed a skill set which allows you to actually do things in the absence of a lot of the modern commercially produced chemistry equipment. Not only
is learning to work with Teflon and glass a useful skill it is also the kind of thing mad science, or Sciencemadness, is all about in my opinion.
I have a small assortment of Teflon rod, sheet and other assorted pieces now from which I can draw on to make a variety of fittings and other
components for mad science type activities. 
Anyway, the information from the last couple of posts is useful whether or not distillation is performed in a commercially produced ground glass, all
glass, apparatus or a homemade distillation apparatus.
[Edited on 12-12-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
confused
Hazard to Others
  
Posts: 244
Registered: 17-3-2013
Location: Singapore
Member Is Offline
Mood: tired
|
|
Quote: Originally posted by cyanureeves  | | also i know my question is not about distillation but it is about DYI. i rigged up a stir em up contraption with a fan and a big ole magnet and it
spins a screw pretty good in a water filled jar. i got it set on a wooden box i made and have to toy with the height or it will spin a vortex until it
makes a toilet flushing sound and acts all stupid. now i need a stir bar so i got a headless screw suspended in some silicone rtv and i'm thinking of
shaving it into shape tommorrow or whenever it cures.i dont know if silicone would be right for an acetone and hypochlorite mixture as in
chloroform,or even hydrazine synthesis.last time i made chloroform i swirled it around myself in freezing weather and that sucked!what can serve as a
stir bar for now until i get some money to buy one? thank you very much. |
Not sure if this will work, just throwing an idea out there, but a paperclip might work
|
|
|
| Pages:
1
2
3 |