| Pages:
1
2
3
4
5
6
7 |
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
119 ml of 40' Baume nitric acid and 105 ml of 1.8 g/ml sulfuric acid were chilled to freezer temperatures, then mixed and chilled to below 10' C. in
an ice water bath on a stirring hot plate.
30 g of pentaerythritol was added in small scoops, allowing each addition to dissolve and temperature to fall back down to 5' C. before next addition.
Temperature ranged from 5 to 8' C. and total time was 30 minutes.
The mix stirred another 10 minutes while a warm water bath was prepared, then temp was raised to 50' C. over another 10 minutes. Mix held at 50' for
20 minutes and drowned in 1 l of ice water. Total time from start to drowning 65 minutes.
Product filtered, washed with distilled water, bicarbonate solution and then more warm distilled water. Returned damp filtered PETN to beaker with 600
ml distilled water and heated with stirring to 60' C. Ph tested and neutralized with 10% ammonia water. PETN filtered, rinsed and dried on paper,
crude PETN was a clumpy and very fine white powder. It resembled the consistency of PETN spilled from cut det cord I have handled.
PETN was dissolved in 270 ml of acetone in covered jar with stiring in hot water bath as temperature was brought to 55' C. 1g each of urea and
bicarbonate were then added. NO AMMONIA WATER WAS ADDED AT THIS TIME as solution tested slightly basic.
Heat was turned off, stir continued on high and dropwise addition of 250 ml of warm water with 2.5 g of dissolved urea started along with 200 g of
crushed ice in small portions.
About 40 minutes to add all ice and water. Mixture was then filtered and rinsed with warm water, sucked as dry as possible in Buchner funnel and dried
on paper.
Some loss past filter occured, and more stuff coming out of solution in the flask of filtrate from recrystalization was noted the next day. The yield
before recrystalization was 64 g. After was 53 g. The apparent dry volume went down by perhaps 1/3 from the crude product after recrystalization.
Consistency of product is odd, matted glittering white needle like crystals. It looks kind of like dry fluffed commercial pyrocellulose- Not dusty,
but not free flowing either. Definitely not a desirable consistency for loading, it pours about like chopped fiberglass insulation. A 100mg dry sample
compressed in Al foil packet and struck briskly between steel surfaces detonates violently, embedding bits of foil into nearby surfaces.
I'd like to get a denser and more pourable product.
[Edited on 31-12-2010 by Bert]
[Edited on 31-12-2010 by Bert]
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
You can. - There are certain issues that if addressed will get you there.....
As long as needles formed you're at least at the nitration stage. The whole purpose of strict attention to QC with the pentaerythritol in industry is
that the paint industry pentaerythritol is SO poor it will at best give a lousy yield let alone fully the proper level of PETN. I have seen very
elegant needles (3mm+) which shaved beautifully into granules. This was nearly always from reagent-level quality and nearly anhydrous HNO3.
The use of nearly anhydrous acid will get the synthesis to that point. Re-crystallization of solid esters is a must. It may test basic to a surface
test but within the crystal you'll have a different story. Mixed acid (from solid alkali metal nitrates) CAN result is fully nitrated end product if
precursor is high quality & temp/time issues are covered well.
The reason I have mentioned the quality of the pentaerythritol at various times was not only the yield but the low quality of paint-grade
pentaerythritol has shown serious problems during war time emergency production.
No needle product will flow. It must be shaved to the 4 um and smaller level and an anti-caking agent used. The most difficult needle product to work
with had always been nitrogunaadine which did not shave what so ever. The most dangerous was MHN which had roughly the same sensitivity as ETN re:
friction / impact initiation. Since Urea was used as stabilizer, strict attention should be paid to decomposition from inside crystal. Surface ph can
be VERY deceptive. The whole purpose of diphenylamine was the discoloration indicator when used as a stabilizer. This CAN be extracted from smokeless
powder with little difficulty.
Industrial acetone will leave some hydrocarbons in finished product. Depending upon levels, shaving may be inhibited by same. Microscopic examination
will tell a story if needles are of inconsistent shape, with, density (400x). Anti-caking agent and light manipulation should shave down to that level
& maintain longevity of product if previous issues are covered in full. Often two re-crystallization are used to achieve higher level of brittle
crystal lattice resulting in excellent shaving through light manipulation. Unless all interior acidic elements removed, shaving process will
accelerated decomposition.
[If you can get it, please] See "The Pentaerythritols" by Berlow.
[Edited on 31-12-2010 by quicksilver]
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Any small scale "shaving" techniques?
|
|
|
User
Hazard to Others
  
Posts: 339
Registered: 7-11-2008
Location: Earth
Member Is Offline
Mood: Passionate
|
|
Another method to obtain a more crystal like structure is recrystallization from toluene.
I get cubic high density crystals this way.
Urbanski wrote some words on this.
Toluene is a very good recrystallization medium for its solubility of petn only at high temps.
If desired you can squeeze out more from the liquid by adding water.
[Edited on 1-1-2011 by User]
What a fine day for chemistry this is.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
I have toluene available, can you point to solubility vs. temperature data?
Downloaded Urbanski years ago, it's on a computer I won't have access to for a couple of weeks though.
|
|
|
Sephi
Harmless

Posts: 3
Registered: 31-8-2010
Location: Italy
Member Is Offline
Mood: No Mood
|
|
et voilà
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Shaving is merely the rubbing, crushing light manipulation of crystalline materials against one another. Often, in certain materials and
circumstances, it happens very naturally. It IS something to practice high safety levels with (obviously) however if the needle is structured trim and
brittle, it will take place simply buy manipulation of the substance from one container to the next, from simple gentle manipulation (for instance in
a static-free bag) can crush to extremely fine powder, most ridged refined crystal forms.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Frankly, the shaveing procedure doesn't sound like it will produce a DENSE product.
What is the recommended mechanism for growing cubic crystals from toluene- Slow cooling with agitation, at a guess?
|
|
|
gnitseretni
Hazard to Others
  
Posts: 283
Registered: 5-1-2007
Location: Colombia
Member Is Offline
Mood: No Mood
|
|
Shaving won't make the crystals denser, it will reduce their size and you will get less/smaller air pockets when you press it and thus a higher
density. At least that's how I understand it.
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Cubic crystals or crushed needles STILL need to be reduced to 2 um or there-abouts to compress so as to eschew air pockets. The point is to reduce
size of particulate (solid).
{see gnitseretni 's comment above.}
Which would be easier to manipulate? There IS a patent to maintain clean cubic crystals in Lead Styphate (Do a search: I posted it several years back)
however the thrust of the patent was for manipulation: to make the product pour into tight fitting containers. To increase density the best possible
method is to liquefy, eliminate bubbles, solidify, etc.
There ARE "trade secrets" to maintaining very TINY and extremely brittle needles that fragments apart into near dust however. That in so far as I am
aware is a temperature re-crystallization scheme.
Keep in mind that solvents in "paint-grade" contain enormous amounts of other materials. Frequently hydrocarbons, that can result in exactly the
opposite of the desired result by leaving behind a sludge of oily contaminants.
The idea of using varying solvents to produce variety in crystal design is very workable but not with kitchen chemistry. The materials must be
appropriately pure. Distillation apparatus is your friend. One thing that should be done with a degree on consistency is when such glassware is first
obtained, is to make several distillations to familiarize oneself with temp control and to have sources of truly pure solvents. Reagent benzene is
commonly sold at close to $200 a gallon at true reagent grade.
|
|
|
DNA
Hazard to Others
  
Posts: 191
Registered: 11-6-2003
Location: @moon
Member Is Offline
Mood: Experimenting
|
|
So just to get this correct, the advantage of having very small particles is that you will be able to get a higher density when loading because there
will be less airpockets.
Higher density means higher VoD, but does the density also correlate to sensitivity, will it be harder to detonate or easier etc?
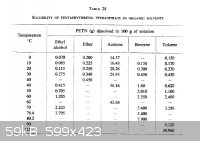
And one comment on the solubility table.
On powerlabs it gives a solubility of PETN of 58g/100mL acetone @ 50*C.
This is confirmed by the following paper:
| Quote: | Solubility of Pentaerythritol Tetranitrate
Robert N. Roberts, Robert H. Dinegar
J. Phys. Chem., 1958, 62 (8), pp 1009–1011
DOI: 10.1021/j150566a032 |
[Edited on 31-1-2011 by DNA]
[Edited on 31-1-2011 by DNA]
Attachment: solubility petn.pdf (344kB)
This file has been downloaded 1275 times
|
|
|
gnitseretni
Hazard to Others
  
Posts: 283
Registered: 5-1-2007
Location: Colombia
Member Is Offline
Mood: No Mood
|
|
Did another PETN synth to try out my homemade addition funnel. Works like a charm so far 
I cast the "hopper" part from aluminum using the lost foam technique. Made a hopper from styrofoam, glued on a sprue and buried it in dry sand and
poured aluminum over it and voila. Not as easy as I make it sound though. That was my 4th casting and even IT didn't completely fill out. But it's
acceptable. The auger is just a long screw, and the motor and gears came out of a printer.
Some pics of the addition funnel:

Direct link: http://img52.imageshack.us/img52/5131/funnel001.jpg

Direct link: http://img820.imageshack.us/img820/6439/funnel002.jpg
Here's a short video of it in action: http://www.youtube.com/watch?v=BrjFfuxVc90
Some more pics:

Direct link: http://img6.imageshack.us/img6/7548/sl731092.jpg

Direct link: http://img573.imageshack.us/img573/8659/sl731093.jpg

Direct link: http://img195.imageshack.us/img195/9282/sl731095.jpg

Direct link: http://img690.imageshack.us/img690/8678/sl731097.jpg

Direct link: http://img822.imageshack.us/img822/2262/sl731098.jpg
232g uncrystallized PETN from 100g PE.

Direct link: http://img715.imageshack.us/img715/4558/sl731102.jpg
[Edited on 7-2-2011 by gnitseretni]
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Interesting little pilot plant you have there.
What is the mixer blade- Stainless steel?
Any feedback control of addition rate? Temperature sensor in the reaction vessel, PID controller... Or is it a pre set constant rate of addition.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
mabuse_
Hazard to Self
 
Posts: 56
Registered: 3-6-2010
Member Is Offline
Mood: No Mood
|
|
That funnel is really cool.
If saved an old mixer for such purposes too. But i wonder how the stirring device will react to HNO3? I would expect it to rust really fast...?
And what's that green stuff? Antifreeze?
|
|
|
gnitseretni
Hazard to Others
  
Posts: 283
Registered: 5-1-2007
Location: Colombia
Member Is Offline
Mood: No Mood
|
|
Addition rate is constant. (About 4g per minute) I tried making a PWM with a 555, and although it worked for a smaller motor, it didn't work with this
one. It does without the gears but not with the gears. Not enough juice I guess.
The mixer blade is some shitty grade of stainless steel, and yes it gets rusty fast. But to clean it just dip it in HCl. I've been wanting to make one
out of aluminum but keep putting it off.
Green stuff is antifreeze, yes.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Quote: Originally posted by User  | Another method to obtain a more crystal like structure is recrystallization from toluene.
I get cubic high density crystals this way.
Urbanski wrote some words on this.
|
User, could you point to which volume/page of Urbanski addresses this? I've had a chance to look at the index of my PDF's but have not located this-
Lots of material to sift through there!
[Edited on 14-2-2011 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Quote: Originally posted by gnitseretni  | Addition rate is constant. (About 4g per minute) I tried making a PWM with a 555, and although it worked for a smaller motor, it didn't work with this
one. It does without the gears but not with the gears. Not enough juice I guess.
|
You may want to consider a CD4536b timer (CMOS type) Fairchild made them. This is an IC and programmable to minutes hours days. A serious little chip,
it handles up to (I believe up to 18-20v so it's not a 555 that has to run as a feed circuit alone to power a motor!).
MANY people experiment w/ the 555 & become discouraged due to having to design two separate circuits but IC's are a vast improvement due to many
being made to handle higher voltage than older active components. It's a very standard 16 pin IC and actually can be pulled from higher powered timer
designs such as a microwave oven timer system. They (CD4536's) and much more modern chips save a great deal of work when designing something of that
nature.
There IS a very modern timer CMOS chip that (relativity) high voltage but I forgot it's model. If you Google cd4536 Fairchild, you'll likely get the
new ones too that are REALLY nice. The 4536 is like the 555 - it cost less than a dollar & are common but the newer ones (IC's) cost more but you
get a great deal more in features.
[Edited on 14-2-2011 by quicksilver]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
That powered auger drive addition funnel / hopper is a neat improvisation.
A variation on the idea could be made from a woodboring auger bit run inside a tube
with a tee section fed by a hopper. This could have a bit longer reach at the discharge end.
This would be handy also for making nitrate additions. A thermocouple sensor and controller setup
could be used to pace the additions based on temperature, interrupting (Not Enabling) the additions
for a selected interval upon temperature rise above a setpoint and resuming (Enabling) additions
on temperature fall to below the setpoint .....basically providing hardware logic "AND" decision function
True?(Enable) / False?(Not Enable) to your timed cycle drive motor. Your addition funnel would then operate
as a thermostat for the exotherm regulating the reaction temperature by rate of addition. Then you would
have a "Thermally Sentient" intelligent powder addition funnel 
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Have you gotten as far as a schematic or a rendering (whole unit) drawing?
I'm getting much more interested in things of that sort. I only wish I had written down ideas I had thought of in the past; as the more I learn about
today's engineering, the more many ideas seem to be within reach.
@ Bert: According to table (above) that solvency is seriously low (& require significant heat). If such a re-crystallization mechanism were to
produce a cubiod it would entail enormous levels of solvent for this to be effective on anything but a lab level. Utilization at 110 C+ doesn't appear
to be an ideal situation.... Common re-crystallization with acetone, when using temperature extremes produce a very fragile crystal. With the widest
possible extremes (the water being close to freezing & acetone being boiling) the fragility of the needle is such that it cannot maintain it's
form in a dry condition with even gentle flow from pouring bottle to bottle. An experimental model of that with an ounce or so would show this to be a
workable method of shaving to an extremely low particulate mass.
Purity of acetone becomes a factor additionally in that often "paint-grade" acetone contains petroleum distillates which would hamper crystal
structure and leave impurities. I have in the past used distilled acetone & the results were different enough to be worth the effort (it's
actually only a few minutes of work as it boils so easily). Distilled benzene is (approx) $200 a gal from Spectrum. I don't like working w/ benzene
too much but found that similar to toluene; it had a high boiling point, thus being more time consuming to get a pure solvent.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
No I haven't drawn a schematic but it should be simple enough to signal process the output a thermocouple input controller like an Omron programmable
PID controller. Basically I was thinking you could output the controller, inverted if necessary, to the reset pin of a 555 timer chip and get the
aforedescribed AND logic function to ENABLE the 555 timer to RUN and cycle through as an on/off astable multivibrator having settable duration on and
off times. The ON output of the 555 timer would drive an optoisolator input
having a triac output to enable / not enable the AC gearmotor turning the auger. So the auger would be rotated by the AC gearmotor for periods
corresponding to the duty cycle settings for the 555 timer. I would have to study the truth table for the 555 because it has been years since I put
chips on perfboard to do whatever.
To do this right would really require that the thermocouple input controller have a setpoint / reset differential capability
(Schmitt trigger adjustable hysteresis) so that for example, if the setpoint is 30C for transitioning the controller output signal, that it must drop
back to below the setpoint temperature, by a user specified amount before "reset" which transitions the controller output back again. So the "hunting
range" for the closed loop can be set at a half degree C or one degree C or one and eight tenths degree C .....whatever differential you specify for
the "reset on temperature fall to value".
To match the thermodynamic curve of a nitration reaction,
there would really be needed a kind of "ramp controller"
added to the timer stage so that each ON cycle of the timer is progressively varied by a user specified percentage from the previous completed ON
cycle. A digital controller that could decrease the ON period by additive tenth / tenths seconds for each successive ON period would be needed
to rate match the process demand so that the oscillations in temperature are dampened from the reagent supply side and the input controller has less
process variation supervision and limiting to do. That ought to be simple enough to implement huh 
|
|
|
gregxy
Hazard to Others
  
Posts: 421
Registered: 26-5-2006
Member Is Offline
Mood: No Mood
|
|
The easy way to create your own controller of this type
is with a micro controller. For example the PIC16F876
which has a 10 bit A-D converter to read the input and
a pulse with modulator that can control a motor.
The rest is done in software. The chips are kind of fun to program. The chip is about 5$ and a board to program the
chip is about $30 on Ebay.
A standard thermocouple would probably not generate enough voltage but there are probably temperature transducer chips
that could be used.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
I am a lazy b*5tard. I just buy a PID controller with appropriate thermocouple, and add a solid state relay if the setup is used for a high current
application. For use in an acid bath you'll need to protect the thermocouple. I use a meat probe type protected with an oversize piece of Teflon
"spaghetti" tube filled with thermo conductive grease, poke the probe into it and keep the open ends above the surface.
http://www.susanminor.org/forums/showthread.php?315-PID-Cont...
You will learn a lot more making it from scratch though. I have a couple of these rigs, one for my bullet casting smelter (high amp, with SSR) and
another for a small heater on my lubricating/sizing press (low amp, no SSR)
http://gunloads.com/castboolits/showthread.php?t=34547&p...
These things learn the response time of your system after a couple of cycles. That could be exciting in a highly exothermic nitration during the first
cycle... Best have a slooooow addition rate and watch it closely.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Yeah I too am all for using the programmable front end and I think some of them do have the programmable differential for reset. Most of them have
some slight
differential anyway simply to prevent rapid oscillation cycling.
Having a timer that will run cycles having a progressively decaying duty cycle
is a cute trick for analog. It would be like a delay windshield wiper that adds a second to the delay time every time it cycles.
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
I know I pulled this a bit off track but I found a great little inverter design with just two power transistors that really could work. There are SO
many decent schematics out there - especially from old Popular Mechanics reprints that this whole concept could be pulled forward into a great design
as all the tough stuff has been shaken out (the basic mechanical design).
An interesting technical question is whether the addition in a given nitration NEEDS (human)augmented timing or whether it would be ideal to make the
timing electro-mechanical?
I know that generally this would depend on some variables (outside temp, etc) & the specific nitration: but from a theoretical ideal: I've thought
this was an interesting issue.
[Edited on 19-2-2011 by quicksilver]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Do your itty bitty baby molecules need nurturing ????  you know ....like people
talk to their plants......as the seedlings break through .....come on little fellows, you can do it you know ....like people
talk to their plants......as the seedlings break through .....come on little fellows, you can do it  Maybe music is the key ..... Maybe music is the key .....
http://www.youtube.com/watch?v=Ty7nf1PBkKM
|
|
|
| Pages:
1
2
3
4
5
6
7 |