| Pages:
1
2
3
4 |
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Yes, it's not easy for the amateur. There are long and detailed discussions about pH control by dann2 and Swede in (I think) the "Thoughts on Anodes"
thread. It is usually achieved using auto syringes or simple dropper systems. As woelen says be careful adding HCL, use dilute acid. One drop of
concentrated acid can drop the solution by several pH units.
I have never bothered with pH control, but then, all my MMO has come free!
Probably more trouble than it's worth.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
I found the post. It is in 'Thoughts on Anodes' and posted 20-12-2009
Its just info. from a patent giving cycle times for cleaning MMO pool chlorinators.
"US Pat. 6391176 is about a pool Chlorinator. The cycle times are shown in the picture of some text below and are of the order of 10's of seconds."
(see the patent or post).
It is quite easy to contoll pH. There is no need for a closed loop controller with a pH probe and an acid doser. Just add acid at the rates suggested
here:
http://www.oxidizing.110mb.com/chlorate/reaction.html
Approx. 0.146ml 12% HCl per amp per hour is what you need in my (and Swede's) experience.
A simple timer and valve is probably the best way to go.
I have used a second hand syringe dosing machine (ebay) it's great for small cells but costs approx. 60 dollars. Acid is worth the bother IMO so long
as you can get acid easily.
Chlorine escapeing from the cell is what causes pH to rise.
Dann2
|
|
|
Jurre10
Harmless

Posts: 17
Registered: 9-1-2011
Location: The Netherlands
Member Is Offline
Mood: Experimenting
|
|
hi,
got my new anodes.
They are verry thick and rough!
My passivated anode is not thick and verry smooth.
I can't remember my other anode ever wass that thick!
Can somebody tell me if there is a way to protect my new andes?
Jurian
If you try your best, but you don't succeed.
Experience is what you need.
|
|
|
Jurre10
Harmless

Posts: 17
Registered: 9-1-2011
Location: The Netherlands
Member Is Offline
Mood: Experimenting
|
|
sorry, double.
chek my last post
[Edited on 15-1-2011 by Jurre10]
If you try your best, but you don't succeed.
Experience is what you need.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: |
Can somebody tell me if there is a way to protect my new andes?
|
You could set up your cell in such a way that the electrode assembly can be lowered into and lifted out of the cell while the PSU is delivering
current so that the electrodes are never inactive in contact with electrolyte.
Of course, that means foregoing cleaning or washing of the anode.
|
|
|
metalresearcher
National Hazard
   
Posts: 757
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
I can't wait for the delivery of the MMO anode so I started another attempt of making KClO3 which appears to be succesfull even with carbon rod
anodes, as the erosion was very low.
www.metallab.net/KClO3.php#exp201101
I made this webpage ten years ago when I made about 300g of KClO3 successfully. Recently I refurbished it and improved the layout and added my results
of last week to it.
[Edited on 2011-1-15 by metalresearcher]
|
|
|
UncleJoe1985
Hazard to Self
 
Posts: 88
Registered: 30-9-2008
Location: Sunnyvale, CA
Member Is Offline
Mood: No Mood
|
|
Good to see I'm not the only one having anode deterioration problems. I've been using an MMO anode I purchased from NorthStar Pyro in a chlorate cell
for 3 weeks. For the first 2 weeks, it was running fine at ~11 amps. During the 3rd week, the current gradually decreased to 5 amps.
I kept adding more NaCl and water throughout to keep the solution saturated and to discourage O2 production.
At first I thought the problem was because I had exhausted most of the Cl ions in the electrolyte. But when I tried the anode in a saturated NaCl
solution, the current only reached ~6A. So, the Cl- concentration isn't the problem. Then I noticed that when the anode dried, it wasn't black
anymore, but covered by a white deposit (see pictures).
I've tried cleaning it with HCl and NaOH, but the desposit remains. What could it be?
I read the Effects of Electrolyte Impurities in Chlorate Cells article markgollum found earlier in this thread. It says calcium and fluoride ions are
both bad. I read the local water report, and it says fluoride concentration is ~1 ppm, which should be safe. However, for ground water, the calcium carbonate % is
310ppm, well past what the arcticle considers acceptable.
So, what could the deposit be? The fact that it doesn't get dissolved and washed away by water suggests it is CaCO3. But I also soaked it
in 15% HCl for 4 hours and the deposit still remains, which doesn't make sense.
Also, I noticed 2 weeks into the electrolysis that even when I disconnect the electrodes so that there's no circuit between anode and cathode, there
is still vigorous bubbling on the anode. Could this be the RuO2 getting reduced (Wikipedia says it's "highly reactive with reducing agents") instead
of getting clogged?

[Edited on 22-2-2015 by UncleJoe1985]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Ruthenium dioxide coatings slowly leach as ruthenates during operation (I recall reading about this somewhere but don't have a reference on hand),
ultimately this leave just TiO2 behind which might explain the light colour. Perhaps such 'spent' anodes can be reimpregnated by painting on a
ruthenium salt solution and then heating to decompose to ruthenium dioxide. Ruthenium's spot price is extremely low of late ($56/ounce), but
purchasing a suitable salt might still be expensive and/or not easy to find?
[Edited on 22-2-2015 by deltaH]
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Unclejoe, can it be some kind of organic precipitation that blocks the current also? Did you try to use some solvents to get rid of that? But what
deltaH says is more true I think..
|
|
|
UncleJoe1985
Hazard to Self
 
Posts: 88
Registered: 30-9-2008
Location: Sunnyvale, CA
Member Is Offline
Mood: No Mood
|
|
| Quote: | | can it be some kind of organic precipitation? |
I also tried cleaning with acetone, but to no effect.
| Quote: | | Ruthenium dioxide coatings slowly leach as ruthenates |
OK, but how can it happen in only 3 weeks? I saw the specs of a pool chlorinator anode and it said the life time was 8000 hours.
So far, the mechanisms I heard about that destroy the MMO coating are:
1. Oxygen evolution, causing the RuO2 to turn into RuO4, which is a liquid.
http://www.waterstarinc.com/files/Resources/White_paper.pdf
2. "caustic accelerated wear from cathode scales" (from Effects of Electrolyte Impurities in Chlorate Cells). It said in one case, 90% of the MMO was
stripped due to hard water scale bridging between anode and cathode.
3. Reducing agents (since RuO2 is an oxidizer)
I still don't know if the white layer is an extra deposit over the MMO or if it's bare titanium. Guess I'll have to sacrifice this anode by scraping
it off and see what's under it 
[Edited on 22-2-2015 by UncleJoe1985]
|
|
|
UncleJoe1985
Hazard to Self
 
Posts: 88
Registered: 30-9-2008
Location: Sunnyvale, CA
Member Is Offline
Mood: No Mood
|
|
I just put the anode under a microscope. I'm pretty sure now the white layer is a deposit, instead of a place where the MMO was stripped away.
I also tried 800 grid sand paper and a screw driver to clean it, but it's not possible without severely chipping off the MMO.
Can anyone identify what the mystery substance that resists all the cleaners I've thrown at it (HCl, NaOH, acetone)?
cleaner, wide angle

cleaner close up 1
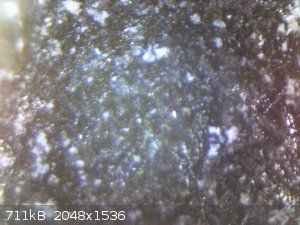
cleaner close up 2
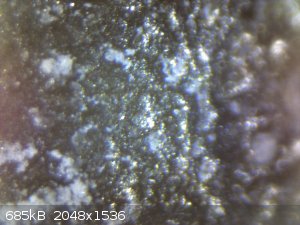
dirty, wide angle

dirty close up 1

dirty close up 2
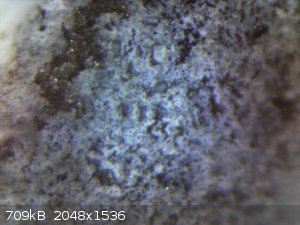
[Edited on 24-2-2015 by UncleJoe1985]
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
TiO2 can be fused with molten NaHSO4 or even NH4HSO4 (it worked for me), however I'm afraid that it'll also dissolve that precious metal oxides by the
way, you could try it on a small spot.
|
|
|
jock88
National Hazard
   
Posts: 505
Registered: 13-12-2012
Member Is Offline
Mood: No Mood
|
|
Try the piece of MMO as a cathode and see if it will work as a cathode. If it will work as a cathode and will not work as an anode (different
directions of current) then you can be sure that it is a layer of Ti oxide that has built up between the Ti and MMO layer that is stopping the
current flowing (as an anode) and not a simple an insulating layer of something else on top of MMO layer (stopping current in both directions).
|
|
|
morganbw
National Hazard
   
Posts: 561
Registered: 23-11-2014
Member Is Offline
Mood: No Mood
|
|
I worked in industry which used Iridium oxide coated plates. They would eventually pacify and the current would not pass and the voltage would
increase ( big time ). Big voltage, low current equals DSA's end of life.
|
|
|
UncleJoe1985
Hazard to Self
 
Posts: 88
Registered: 30-9-2008
Location: Sunnyvale, CA
Member Is Offline
Mood: No Mood
|
|
Victory! My anodes have been resurrected! 
It was quite simple - just soaking the anodes in an initial 5% solution of NaOH at 80C for 2 hours. Although tried NaOH before, I missed the
importance of high temperature and time.
current measurements (5V, saturated NaCl solution)
new: ~10 A
dirty: ~2 A
cleaned: 4.5 A
I only did a crude current test and probably didn't have good electrical contact and had a larger distance between electrodes than in the actual cell.
So the current might be bigger than 5 A. However, I'm expecting a large drop from 10A due to my earlier abusive cleaning efforts.
That anode is moot since I'm using a much bigger one now:

It only took 1 month for this anode to passivate (go from 18 amps to 5 amps)! I cleaned it the same way and was able to get 9 amps (again, it's
probably higher due to the crude testing).
Here are some photos of the smaller, cleaned anode.
You should see a big difference compared to my earlier photos
after cleaning

after cleaning, 4x zoom

after cleaning, 54x zoom
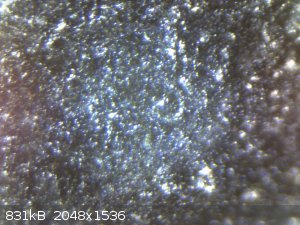
My conclusion?
It's the wrath of calcium silicate! 
The Morton salt I'm using has calcium silicates to prevent caking. I don't know much about silicates but it seems they're glass like and insoluble.
But I suppose the basic pH of most amateur cells could dissolve trace amounts of it. And since molten NaOH will utterly dissolve glass, it totally
makes sense that a hot solution of NaOH will dissolve the deposits.
I received tremendous help from the "Effects of Electrolyte Impurities in Chlorate Cells" by Richard A. Kus referenced earlier in this thread. It
mentioned silica in several places. Appreciate it.
It would be great if someone else can try this and report how much it helps.
[Edited on 3-6-2015 by UncleJoe1985]
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Congratulate me! I run exactly into the same situation as UncleJoe1985, look at his pictures of the anode before cleaning - looks same as mine,
terrible.
Not sure if it changed voltage/amperage very much (didn't measure carefully before and after), I ran it for 5 days at 4Amps and 4.4volts (surface area
both sides: 100cm^2 of mesh) and got what I got - coating of white stuff on MMO. But I have a clue what this might be in my case - SnO2! Because I
wanted to test cathode material from 60/40 Pb/Sn solder and it actually holds up the hot brine very well (even when not under cathodic protection),
however it seems some Sn leached out into alkaline solution and deposited on the anode! It also deposited on the walls of the glass container and I
can rub it off with my finger, but on anode is different - it's very rough, still possible to clean mechanically. I had to use Pb cathode instead of
Pb/Sn solder, might do a good cathode as well (corrosion resistant, what do you think?), any suggestions how do I clean the surface from what is
supposedly SnO2?
[Edited on 13-6-2015 by papaya]
|
|
|
UncleJoe1985
Hazard to Self
 
Posts: 88
Registered: 30-9-2008
Location: Sunnyvale, CA
Member Is Offline
Mood: No Mood
|
|
I don't think having SnO2 on your anode will cause passivation since it's quite conductive with a resistivity of 2 * 10^-5 ohm * m (ref. 1)
or 1.1 * 10^-7 to 7.2 * 10^-6 (ref. 2)
1. from Handbook of Transparent Conductors, p. 157
2. Effects of Substrate Temp. on Structural Properties of Tin Oxide Films produced by Plasma oxidation after thermal evaporation
After all, tin compounds should have many similar characteristics as their lead counterparts and PbO2 is very conductive.
I think a general rule is that acids will turn metal oxides into soluble salts.
CaO + 2 HCl -> CaCl2 + H2O
So HCl might work (but can it also destroy the precious RuO2 and IrO2 too?). The Wiki for SnO2 also says strong
alkali solutions will dissolve it.
Why would you ever want to use tin or lead for the cathode considering how flimsy it is? For chlorate cells, titanium is the best:
http://www.oocities.org/capecanaveral/campus/5361/chlorate/c...
[Edited on 19-6-2015 by UncleJoe1985]
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Conductivity of metal oxides depends on many things, such as how they are prepared. Most of them are not prepared by "wet" methods (except PbO2
maybe), but with some kind of pyrolysis. Also what deposited on my anode given under what conditions it happened is more likely to be stannic acid or
SnO2.xH2O, which is not conductive. I tried 5% HCl but that acid changed color very quickly to some yellow-greenish and I stopped at that point since
I thought this somehow may be related to leaching of RuO2. I tried nitric acid, but it had no noticable effect. Then I tried boiling NaOH and it
finally did the job (in few hours), however after that when rubbed with a finger a black stain could be noticed, so some % of coating is lost. Anyway
I tested that anode and compared with a similar new one - there's no difference in currents (at fixed voltage), and there's no difference in
"overpotential" as long as I can tell (from the minimum voltage vallues at which electrolysis only starts at few milliamps).
Why use Pb for the cathode? First, it's very resistant to corrosion (even when the cell is off), second you can take a copper foil, cut whatever shape
you want and then apply solder (or Pb) and that thin layer will last! However after this accident I'll not try Sn/Pb solder in the future, maybe pure
Pb can still do the job. Also I want to mention that I got very low current efficiency for my chlorate - about 25% percent only (not using dichromate
and Sn/Pb solder as a cathode), which may be related to higer hydroden evolution overpotential for this metal (Pb is almost the same as Hg in this
manner), so yes, all I did is purely experimental and not to any use yet. Now I'm trying with some type of stainless steel, which turned out to be
very robust in this electrolyte, next maybe will try cupronickel or melchiour if it's tight enough.
[Edited on 19-6-2015 by papaya]
|
|
|
UncleJoe1985
Hazard to Self
 
Posts: 88
Registered: 30-9-2008
Location: Sunnyvale, CA
Member Is Offline
Mood: No Mood
|
|
Congratulations on restoring your anodes. Feels very relieving.
I don't want to reduce your curiosity, but that site (made by this site's dann2 I think) clearly says titanium has been the cathode material of
choice for (per)chlorate cells because
| Quote: | | Using Titanium metal stops the reduction of Chlorate unlike Iron which reduces Chlorate |
| Quote: | | may be related to higer hydroden evolution overpotential for this metal |
I was thinking the same thing. I found that to be a problem too when making lead acetate electrolytically
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Titanium is very good of course, but it's hard to me to work mechanically with it without suitable tools - it's very tough and not that easy to cut,
then welding is impossible without inert gas, other problems. And chlorate reduction is not the major problem as far as I remember correctly, worse
problem is hypochlorite reduction which takes place on all types of cathodes (unless dichromate additive is in there). Stainless steel shows
unexpected stability btw - it doesn't rust even at the places above liquid layer, only minor blackening effect is observed. But the steel also is very
hard to work with.
|
|
|
jock88
National Hazard
   
Posts: 505
Registered: 13-12-2012
Member Is Offline
Mood: No Mood
|
|
Grade one, two, three and four Ti are easy to work. They are soft. About the same is mild steel.
It can be spot welded easily.
I think that is would be fair to assum that Ti reduces hypo. less than Iron. By how much is not known.
Stainless steel is hard. Someone suggested that there may be a layer of 'Chromate something' on stainless steel that may reduce or eliminate
reduction.
There are many types of ss as far as corrosion is concerned.
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
I reverse my anodes in sodium hydroxide at 3.3V for 12 hours and aim for a current <300mA, the two small bits I originally cut from larger ones are
still going strong, but I dont take mine above around 3.5A as i dont see the point of very high currents. I also swap the anode and cathode every
other run but I dont do many and only small runs
Dont ask me, I only know enough to be dangerous
|
|
|
Simoski
Hazard to Self
 
Posts: 82
Registered: 24-12-2017
Location: Johannesburg South Africa
Member Is Offline
Mood: No Mood
|
|
Hi
My postassium chlorate cell has been running great for months now, but all of a sudden chlorate is crystalising all over the anode to the point that
its passivated.
Do you think I should add water or more KCL?
I get the feeling that its just super saturated with chlorate and at night when it cools the chlorate crystalises on the anode...
any ideas?
|
|
|
markx
National Hazard
   
Posts: 646
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
Quote: Originally posted by Simoski  | Hi
My postassium chlorate cell has been running great for months now, but all of a sudden chlorate is crystalising all over the anode to the point that
its passivated.
Do you think I should add water or more KCL?
I get the feeling that its just super saturated with chlorate and at night when it cools the chlorate crystalises on the anode...
any ideas? |
Harvest the crop and resaturate with chloride.....no point in trying to run it dry. You risk damaging your anode (MMO or platinum plated) by running
at a very low chloride concentration.
Exact science is a figment of imagination.......
|
|
|
Simoski
Hazard to Self
 
Posts: 82
Registered: 24-12-2017
Location: Johannesburg South Africa
Member Is Offline
Mood: No Mood
|
|
ok will do Markx, thank you
[Edited on 16-2-2019 by Simoski]
|
|
|
| Pages:
1
2
3
4 |