| Pages:
1
..
27
28
29
30
31
..
60 |
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by garage chemist  |
Just some other experiment I found: a demonstration experiment to show that bones contain phosphorus.
It is from a german site, the adress is here:
http://cc.upb.de/studienarbeiten/seidel/allgem_chem/versuche...
Translation of the important part:
Cleaned, boiled and dried chicken bones are burned with a bunsen burner on a fireproof surface and directly heated with the flame until they have
turned into white ash.
2g of this bone ash are mixed with 0,5g magnesium powder and 0,5g kieselgur.
The mix is heated in a test tube which is plugged with a glasswool plug. After the reaction has finished, it is left to cool and the glasswool plug is
removed in a darkened room and observed closely.
A glow is visible on the glasswool.
When the residue in the test tube is mixed with water, gas bubbles are evolved which self-ignite on contact with air. They are phosphine.
Reactions are on the site that I posted.
The important feature here is the use of magnesium instead of the often- used aluminium. Mg reacts at a much lower temperature than Al.
The SiO2 must be finely dispersed in the mix, hence the use of kieselgur. Quartz sand is not fine enough, even after good grinding.
The reaction of calcium phosphate with Mg is very exothermic.
Good heat is still necessary though.
The following reaction, the reaction of the phosphide with SiO2, is the actual phosphorus producing reaction, and the important bottleneck in DIY P
manufacture.
The use of kieselgur promotes the reaction by increasing contact area due to its fine microporous structure.
The ingredients should also be grinded together very well, due to this reason (dont use too fine Mg powder though, otherwise it might be more of a
pyrotechnic mix. But the SiO2 and bone ash should be intimately mixed.). |
according to garage chemist this would indicate that the use of SiO2 is an absolute necessity in this reaction. i have been using silica sand. i
wonder if this is a hindrance and i should perhaps acquire some silica flour? i am not as concerned over the use of Al vs Mg since temp is not a major
issue for me but the finer SiO2 may be a link to increasing the yield. thoughts on this musing?
|
|
|
jgourlay
Hazard to Others
  
Posts: 249
Registered: 9-7-2008
Member Is Offline
Mood: No Mood
|
|
Would silica flour be a good substitute for Diatomacious earth?
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Yes, most likely.
|
|
|
redfox87
Harmless

Posts: 7
Registered: 18-10-2010
Member Is Offline
Mood: No Mood
|
|
I was curious if Charcoal would burn sufficiently hot for the Calcium phosphate method?
" Charcoal may be used to smelt a variety of metals from aluminum to copper as it burns at the necessary temperature: 1,100 °C (2,010 °F)."
I realize thats around the threshold, and am uncertain if it is just enough or just a bit short.
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 860
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
If you blow air through the burning coal in a proper fashion, it can melt iron.
I speak from my experience with metalsmithing.
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
charcoal briquettes have other chemicals in them that make them suspect for this reaction. you would be better to use natural wood charcoal. coal is
carbon and carbon (coke) is what they use in industry along with calcium phosphate and silica. the problem is that with most setups available at home,
the temp needed to run the reaction is out of reach. you need a pretty strong furnace and a retort capable of surviving it. the sodium
hexametaphosphate with aluminum reducing agent and silica sand or silica flour have been the most viable route so far as it has shown some success.
polverone, blogfast, magpie and others made this discovery years ago in this thread. i recently scaled up their efforts and had some decent success.
these reagents are cheap and plentiful and perform fairly well. the industrial method with carbon and calcium phosphate uses temperatures in excess of
1500C. see my youtube channel for a detailed process.
http://www.youtube.com/user/rogeryermaw
|
|
|
redfox87
Harmless

Posts: 7
Registered: 18-10-2010
Member Is Offline
Mood: No Mood
|
|
Your videos are appreciated!
I had another thought. Could a graphite crucible be used to ignite thermite with calcium phosphate. Then pipe the vapors using a graphite pipe
(graphite rod drilled to make a tube) to a ice bath to collect phosphorous. What kind of problems might this method encounter. I think this could
be the quickest route to elemental phosphorous and could probably be scaled up fairly easily or perhaps a valve to further add more calcium phosphate
to the reaction as it proceeds.
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
| Quote: | | What kind of problems might this method encounter. |
For starters, making a whole working assembly out of graphite is not as easy as you make it sound. You speak of a crucible and a single tube. Even
assuming that your 'crucible' is actually a hollow graphite vessel with a suitable opening, and that you have some way of making a gas-tight
connection between two graphite pieces, I wonder whether you think this tube points up or down. Briefly, it needs to be bent, so that the vapors
travel up out of the melt and then down under the water's surface. And graphite (for our purposes here, I don't need to hear about graphite fiber
sports equipment) doesn't bend. Of course you can get around this by assembling multiple tubes and a machined elbow, again assuming you have some way
of joining things properly. Graphite, for what it's worth, is not trivial to work, and produces very fine dust that must be dealt with. In short your
proposal is either very expensive (if you plan to order all these parts made to order) or else requires extensive special expertise (and even then the
graphite blanks are not cheap compared to steel tubing).
| Quote: | | could probably be scaled up fairly easily |
Yes, because when I want to move up from a 4" outside diameter graphite reaction vessel to 8"all I need to do is order a $400 graphite blank and then
somehow machine out a hollow within it. You want something that scales easily? Design around cheap materials like steel and firebrick. Also, thermite
as a heat source is not something I would associate with scalability.
| Quote: | | add more calcium phosphate to the reaction as it proceeds. |
And the heat to decompose this new material will come from where? Also, despite the outgassing of various products, I don't think in general that the
reaction frees up a lot of space in the reaction vessel. And while adding more phosphate to a very hot vessel without also letting in air or letting
out toxic gas is not something that would be a problem industrially, it's not all that easy to rig it up at home.
| Quote: | | I think this could be the quickest route to elemental phosphorous |
Well, you're certainly welcome to demonstrate it if you want to have a go. Sounds fanciful to me.
|
|
|
redfox87
Harmless

Posts: 7
Registered: 18-10-2010
Member Is Offline
Mood: No Mood
|
|
graphite crucibles are very cheap...
graphite rods are very cheap...
It does not need to be air tight for the connection, it simply needs to provide the path of least resistance. Graphite piping is not necessary, but I
thought it provided the best material to withstand the high temperatures of the initial vapors. I'm sure a metal condenser(water cooled or otherwise)
would work fine, and I don't think the vapors are going to be near as hot as the temperature of the thermite reaction.
also if the graphite pieces were clamped together with sufficient force I would think the crystal lattices being identical would stack fairly nicely
to make a decent seal (perhaps not air tight but close enough). As for scaling up I don't see why a wider diameter would be needed, why not just a
deeper cavity?
The heat to react more calcium phosphate will be at the site of the boiling aluminum perhaps, or did all that heat just disappear as soon as the first
round of calcium phosphate was vaporized (I would not think so). Perhaps adding more calcium phosphate would prove problematic, so the simple answer
is don't add more. If the reaction is efficient and cheap just run it again.
As for the gasses. The gasses would be released through the water of course and if addition of further gases was desirable an inlet could be rigged
up easily. Perhaps run an inlet tube through the exhaust pipeline.
Spending 50 bucks on graphite(it is MUCH cheaper then you make it sound) and making some thermite for a few bucks seems far less fanciful then feeding
an expensive arc or tube furnace with large amounts of gas.
I suppose my question on the matter would be will the vapors from this reaction melt steel. Are there any propositions for alternative piping
methods. My main concern is the temperature of thermite, I do not want to melt any piece of the apparatus, so some scheme or knowledge to ensure this
does not happen is essential. I think steel piping might be adequate if it is adequately far enough from where the thermite is igniting. Perhaps a
coolant gas (one of the nobles) could be utilized for this purpose.
Now your going to make me shell out to try and prove a point lol. Might take me a few weeks but I promise I will get around to this.
[Edited on 23-10-2010 by redfox87]
[Edited on 23-10-2010 by redfox87]
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
personally i'm dying to see your results. yield notwithstanding, i think my 6 dollar and change retort kicks the shit out of any other idea i have
tried so far. i hold all the ideas i have seen in this thread in high regard but for efficiency,and reproducible, that little cheap ass wood furnace
is doing well. even if i had to buy everything i have used new, and specifically for this project, considering how many runs it has survived, no
competition for cheapness and simplicity. the major flaw with graphite is that it IS expensive. one thing you should consider is that success requires
experimentation and a $50 piece of graphite is not what i would call expendable. will it survive the heat? will it survive chipping the slag out of it
for successive runs? every time you have to move it and take it apart will you damage the joints? what i'm using may not be ideal but it is cheap and
expendable and i have at least 18 grams of white nasty from it. personally, i think the "best" method for home use would be a steel tube retort of
heavy wall construction and an induction heater.
for those of you waiting on the latest result, sorry it took so long but i have been ill and suffering some truly fucked up allergy attacks at the
same time. i think my guinea pigs are getting to me...well their hay anyway. going to try orchard grass and see if it helps. i hope so because getting
rid of them is not an option. i love those little boogers! on top of that i gave myself the worst headache the other day...got a little dab of
nitroglycerin on my arm and now i know what a migraine feels like. NEVER AGAIN!!! i'm not big into pyro anyway. just having a bit of fun which i paid
for
i have some small data. the usual measurements. roughly 6.5 grams of output. longer down pipe may be helpful but deeper submersion is ill advised. it
may not be a problem normally, but if for any reason you should have to turn your back on your furnace for any length of time, suckback will ruin your
chances of good yield. steam explosion no but wet reactants and subsequent phosphine yes. sorry for the poor video but it was around 2:30 am, pitch
black and i am a bit tired:
<object width="480" height="385"><param name="movie"
value="http://www.youtube.com/v/_rr8MPN0sBg?fs=1&hl=en_US"></param><param name="allowFullScreen"
value="true"></param><param name="allowscriptaccess" value="always"></param><embed
src="http://www.youtube.com/v/_rr8MPN0sBg?fs=1&hl=en_US" type="application/x-shockwave-flash" allowscriptaccess="always"
allowfullscreen="true" width="480" height="385"></embed></object>
those of you on the phosphorus path have seen phosphine and know what you are looking at. ignore the flying cinders...i do and they're flying at me!
however, through the collector, you can see the yellow, orange flashes. obvious phosphine. the more greenish ones are escaping phosphorus.
what this leads me to believe is that to truly increase yields, this will have to be done with a LONG temperature controlled condenser that will drop
P in its liquid state into an oxygen free collector of water cool enough to solidify the goo as it drops. otherwise there will be losses. and having
an oxygen free space for it to drop between the condenser and the collector will eliminate any possible suction issue. conversely, those of you i know
i can trust can send me a few bucks for a new retort and i would be glad to produce it for you. have to ship overnight to reduce prying eyes. of
course this applies to the u.s. since 1. getting it out of the country would require an intrepid soul who is quite liberal with his anus and 2. most
other countries can purchase red P (if even with some difficulty) and convert it.
[Edited on 26-10-2010 by Rogeryermaw]
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
The advantage of phosphoric acid and activated carbon heated by microwaves and contained in borosilicate glass, fused quartz, or ceramic is that there
would be no slag, just CO and P.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Roger:
Hear, hear!
The main improvement of your apparatus would actually be (apart from the collector problem) an easier and cleaner heating method. Propane is still an
option but propane furnace design to reach the right temperatures will not be so easy.
Microwave heating remains for this purpose and pardon the pun, a 'pipe dream'... ;-)
|
|
|
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
With forced air (a $10 air mattress inflater) I get 1400oC easily with a propane furnace even when using cheap autoclaved cell concrete (for inner
walls) as refractory.
How I did it:
www.metallab.net/ytongfurnace.php
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
i have to wonder...if one were to use a graphite crucible...would it not become part of the reaction?
|
|
|
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
A graphite crucible is what I use for melting metals. But for other things such as distilling off P4 you can use the same vessel as in your wood
fueled furnace.
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
what i mean is, since carbon is used in the industrial production of phosphorus, were you to use a graphite crucible, would it not react when the
other reactants start and the temp spikes? when the reaction starts, temp rises in the retort. would this not cause the graphite(carbon)to partially
react as well? wondering if this possibility makes the use of a carbon based vessel less feasible...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Rogeryermaw  | | what i mean is, since carbon is used in the industrial production of phosphorus, were you to use a graphite crucible, would it not react when the
other reactants start and the temp spikes? when the reaction starts, temp rises in the retort. would this not cause the graphite(carbon)to partially
react as well? wondering if this possibility makes the use of a carbon based vessel less feasible... |
It would, superficially, but that wouldn't matter. They use graphite crucibles for melting gold (get them on ebay quite cheaply) and these crucibles
also slowly burn away due to oxygen in the furnace. It limits the lifespan of the crucible, that's all...
But there's no need for graphite here: good old steel does the job. If it ain't broke, don't fix it!
[Edited on 26-10-2010 by blogfast25]
|
|
|
redfox87
Harmless

Posts: 7
Registered: 18-10-2010
Member Is Offline
Mood: No Mood
|
|
The issue with the microwave is that your going to have some issues with the phosphoric acid eating at your glassware.
I tried the microwave with nalgene reaction vessels and that failed quickly as the nalgene melted quickly.
Graphite should remain largely un-reactive, and its high melting temp is really attractive, it is only attractive for an experiment with something
like thermite though as its an excellent insulator and will not transmit heat very well.
I was thinking about the use of a graphite crucible. What if one used a graphite crucible, had two lines feeding in oxygen and propane, utilizing an
exhaust for the P4 to be cooled. Would this be viable or would the direct contact of the oxidizing gas' prove to be a problem.
If that didn't work perhaps an iron or steel container within the graphite crucible with the gas' burning. (the crucible would be used as a heat
shield mainly). I used to blow chemical glassware and am aware that by adjusting the ratio of propane to oxygen you can essentially adjust the
temperature of the flame, meaning an ideal temperature that would not be detrimental to the reaction vessel could be calibrated. Perhaps I'll just
get a larger pipe to place the smaller pipe in to ensure I do not lose that oh so precious heat to the atmosphere.
I will work on gathering reagents for my experiment and keep the board posted. I'll try to record it, so whether it be success or failure it will be
an interesting watch.
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
i really do think blogfast and metalresearcher are onto something there. graphite is all well and good but i think a furnace of refractory brick made
to fit with less than an inch or so of space around a heavy walled steel reaction vessel, with propane injection and forced air may be just the
ticket. blogfast i am wondering about the use of a flux in this reaction...it seems to help with the speed of the process overall but from looking at
the product post reaction...it may either be contaminating the phosphorus or causing some of it to come over as red P. i will show some comparison
pics by tomorrow to illustrate what i mean and solicit opinions. the products of the reactions with salt have been darker than my earlier attempts.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Rogeryermaw  | | i really do think blogfast and metalresearcher are onto something there. graphite is all well and good but i think a furnace of refractory brick made
to fit with less than an inch or so of space around a heavy walled steel reaction vessel, with propane injection and forced air may be just the
ticket. blogfast i am wondering about the use of a flux in this reaction...it seems to help with the speed of the process overall but from looking at
the product post reaction...it may either be contaminating the phosphorus or causing some of it to come over as red P. i will show some comparison
pics by tomorrow to illustrate what i mean and solicit opinions. the products of the reactions with salt have been darker than my earlier attempts.
|
Roger:
Have you ever seen melted salt? It loses salt (vapour) like water loses water vapour! So it's very likely some will distil over with the P. Of course
it also has a higher MP and BP, so it condenses and resolidifies before the P. But your P is very likely quite badly contaminated with all sorts of
things and strictly speaking needs a purifying distillation (good luck with that!) to get to decent purity...
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
You may be getting phosphorus chlorides and oxychlorides using NaCl as flux. Heating P2O5 and NaCl was a mentioned production method in other
threads.
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
here's a look at the color differences:

no flux

with flux
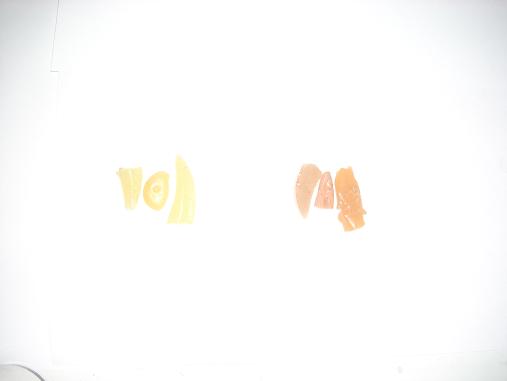
side by side
pretty huge difference huh? it shouldn't be too hard to re-distill these with an inert test tube large enough. i did some messing around with making
the trichloride and found that CO2 is suitable for an inert atmosphere so it should be doable. how hard is it to remove the phosphor coating from
fluorescent tubes? perhaps i can bend one of these into a suitable vessel for the job...
[Edited on 30-10-2010 by Rogeryermaw]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Rogeryermaw  |
pretty huge difference huh? it shouldn't be too hard to re-distill these with an inert test tube large enough. i did some messing around with making
the trichloride and found that CO2 is suitable for an inert atmosphere so it should be doable. how hard is it to remove the phosphor coating from
fluorescent tubes? perhaps i can bend one of these into a suitable vessel for the job...
[Edited on 30-10-2010 by Rogeryermaw] |
No, not huge, but certainly significant, I'd say.
Getting the phosphor off TL tubes wouldn't be the problem, but the glass wouldn't be suitable AT ALL: too thin, too brittle and not borosilicate.
Forget trying to bend it.
If you're venturing into distillation of either P or PXn, you'll need a small 'professional' boroglass still for safety. And vacuum or argon (I
wouldn't trust CO2 with P: too reactive...)
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
how about a borosilicate retort?

i happen to have a couple of these. one hole stopper in the top with glass tube to run nitro or argon perhaps? i also have some distillation equipment
and smaller flasks that may be easier to heat evenly but i hesitate to run P gas through equipment with joints that may freeze with solidified
product. then to find a suitable heating fluid in which P is not soluble in case of a glass failure. water is out due to inability to reach the BP of
P. P is somewhat soluble in most oils. solvents are mostly too volatile for the job and who wants to heat a solvent like that anyway...melon
scratcher...
woelen performed a distillation of phosphorus under CO2 atmosphere with no ill effects as seen on his website here:
http://81.207.88.128/science/chem/exps/RedP2WhiteP/index.html
this is what prompted me to use CO2 to inert the apparatus to re-distill this white P. for some reason it's not appearing as a link so just
copy-n-paste it i suppose.
[Edited on 31-10-2010 by Rogeryermaw]
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Bah, my intensive decorating means I've lost out on following this, so I need to have a back read.
But, in terms of purification, wouldn't sublimation be a better plan than distillation? Less glass involved (so less chance of it breaking / money
being lost), do it under vacuum (squirt some inert gas in there first as well if you have some) to reduce the fire problem, no fractioning needed.
Probably won't be super pure given the lack of fractionation, but it should get it most of the trace gunk out.
{edit}Depends what you plan to do with it downstream as well. If you're going to be making phosphorus compounds with it, it may be far easier to go
straight to those with the impure phosphorus and then purify the resulting product, which may be better behaved (boiling at lower temperatures, not
bursting into flames and so on). The transition to the product, depending on what is it, may also help clean it up with much less involved methods;
e.g. the contaminants may not even interact with the reagents and so will more easily get left behind as solids in a liquid (filtration) and so on. If
the goal is the trichloride, using hydrochloric for that will produce phosphoric acid, so you'd need to be using chlorine (hydrochloric on manganese
dioxide). Then some pure oxygen to go to POCl3. A respirator and some practice with gas handling / scrubbing / neutralization would be a good idea for
those bastards. Definitely want the kids at school or asleep for that one. 
[Edited on 31-10-2010 by peach]
|
|
|
| Pages:
1
..
27
28
29
30
31
..
60 |