| Pages:
1
2 |
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Dissolving Bismuth, the road to Sodium Bismuthate, and other uses.
Bismuth, available relatively pure as environmentally friendly shot for shooting, some sinkers, and those neat looking crystals from rock shops, or,
of course eBay and other Internet sources.
I had some bismuth as pieces from a larger bismuth crystal that I got from a mineral shop and decided that it was time to do something with it like
make sodium bismuthate. Looking up some solubility I quickly plotted my course:
Bi(s) + 3HCl(aq) ---> BiCl3(aq) + 1 1/2 H2(g)
BiCl3(aq) + 3KOH(aq) ----> Bi(OH)3(s) + 3KCl(aq)
2Bi(OH)3(s) ---[Heat]---> Bi2O3(s) + 3H2O(g)
Bi2O3(s) ---[NaOH(l)]---> 2NaBiO3(s)
Purify by recrystalization.
However the first step got me right off. Maybe it was due to the fact that the bismuth I had was from a large crystal. But when added to HCl there
was some minimal bubbling in the beginning then nothing, no noticeable change a day later. So I looked up if Bi2O3 was soluble in acid and indeed it
is, so I added some 30% H2O2 to speed things up. No luck, it just oxidized the chlorine and made chlorine gas. So I added some 95% H2SO4, hoping to
both increase the strength of the solution by dehydrating it and create Caro's acid in situ, didn't help noticeably. Next up, added some
70% nitric, didn't help much, so I added some more and all at once it started to dissolve readily. The next day a majority of the bismuth was in
solution, so bismuth, at least in this crystal form, is a pain to dissolve.
So today was the fun part, adding KOH to the solution till basic and the Bi(OH)3 precipitated out. Total volume of concentrated acid was 200ml  And only about 3 - 4 g of bismuth was dissolved in it. So needless to say it took
awhile and heated up, and boiled, because I was adding solid KOH due to the fact that I was using my largest beaker with only a 300 ml volume. About
50g of KOH later, the yellow solution became colloidal but was still acid to luminous. Added more KOH and all at once the precipitate turned cream
color and was now distinctly basic, pH > 14. The precipitate was settling rapidly when I put away the mixture for today. Tomorrow, filter and
wash with dilute KOH and possibly heat off the water. And only about 3 - 4 g of bismuth was dissolved in it. So needless to say it took
awhile and heated up, and boiled, because I was adding solid KOH due to the fact that I was using my largest beaker with only a 300 ml volume. About
50g of KOH later, the yellow solution became colloidal but was still acid to luminous. Added more KOH and all at once the precipitate turned cream
color and was now distinctly basic, pH > 14. The precipitate was settling rapidly when I put away the mixture for today. Tomorrow, filter and
wash with dilute KOH and possibly heat off the water.
The Bi(V)/Bi(III) oxidation potential is estimated at 2.03 V making it one of the most powerful oxidizers in aqueous solution, lying between
peroxodisulfate and ozone in terms of power. Sodium bismuthate is a yellow-brown solid that is stable in the absence of water but is somewhat
hygroscopic, it will keep in a .5 M HClO4 solution for several days with minimal decomposition. Decomposes in hot water forming bismuth oxide and
sodium hydroxide, and liberating oxygen.
Example of a reaction of sodium bismuthate oxidizing Mn2+ to permanganate:
2Mn2+ + 14H+ + 5NaBiO3 --> 5Bi3+ + 5Na+ + 7H2O + 2MnO4
Does anyone have any other uses for the interesting element that is bismuth? Or an easier way to dissolve it? I will post further here as I get more
into the preparation of sodium bismuthate.
MSDS sheet from which some of the bismuthate information was taken.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Highlights from Mellor: "Bi is but slightly attacked by HCl, hot, cold, dilute, or concentrated. It is very sparingly soluble in hot H2SO4
forming Bi2(SO4)3 and SO2. Bi is readily attacked by dilute and conc. HNO3: 2Bi + 8HNO3 = 2Bi(NO3)3 + 4H2O + 2NO. The nitrate dissolves in aqua regia
forming BiCl3.
If Cl2 is passed into a boiling aqueous solution of KOH in which Bi2O3 is suspended, a dark chocolate-brown precipitate is formed. The precipitate is
washed with water, dilute HNO3, and dried at 180°. The precipitate appears to be a mixture of the pentoxide and tetroxide. The greater the excess and
concentration of the lye, the greater the yield of the tetroxide. The tetroxide, more or less contaminated with other oxides, is formed when the
trioxide is oxidized with K ferricyanide in alkaline solutions.
If the current of Cl2 is continued until the precipitate becomes scarlet red, the washed precipitate has approximately the composition KBiO3. If the
precipitate is washed and boiled for a short time in dilute HNO3, scarlet red metabismuthic acid is obtained." He goes on to mention, among other
things, that 15% NaOH + Bi2O5 gives NaBiO3.
I don't see anything anywhere on trioxide/NaOH/air/no electricity giving the bismuthate, instead of heating with Na2O2, but my home library is
limited.
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Bromic acid; I would be interested to see you balance the equation for the reaction of Bi2O3 and NaOH to give NaBiO3. (OK, I know you meant to add the
O2 as well, but there are some less experienced folks who read this and would get puzzled)
S C Wack
I've got a copy of Mellor too (at least, the library at work has) and it's a very useful reference, but I don't believe in a tetroxide
for a Bi. You could talk me into a pentoxide without too much trouble and I think we can take the sesquioxide as read, but I will need some convincing
about a stable even oxidation state (except zero which, as bromic acid noticed, is quite stable).
[Edited on 31-5-2004 by unionised]
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
From Merck: Bismuth tetroxide[12048-50-9]: Gmelin's, vol19, 115 (1927).
Handbook of Preparative Inorganic Chemistry vol1, 628 (1963).
I doubt that they argue for the existence of Bi(IV). I would think that they explain it as the Bi's bonded together, with 2 O's on each Bi.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
In Brauer it states that Bi2O3 is oxidised with Br2 according to
Bi2O3 + 6 NaOH + 2 Br2 -> 2 NaBiO3 + 4 NaBr + 3 H2O
I wouldnt know whether oxygen is sufficient... but given the oxidising power of bromine...I better not guess, I couldnt tell ...
In Brauer it also states that Bi2O3 with HCl (aq) forms BiCl3 -but it emphasises to dry this in a CO2 atmosphere - suggesting (to me) that that
oxygen will disturb the reaction. Sounds tricky to me. (the thing is, apparently BiCl3 when mixed with H2O will form BiOCl, but only if the excess of
H2O/HCl is great)
And yes, Bi is most readily dissolved in HNO3, proportions such as 10 g Bi and 25 ml 65% HNO3 work well.
Edit: Sorry SC Wack I didnt realise until just now that my post overlaps in part with yours 
[Edited on 1-6-2004 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Please excuse the ranting and circular logic...
Thank you S.C. Wack for all your information, I have some references on manufacture of potassium bismuthate and such using Cl2 with KOH(aq) as an
oxidizing agent but this is quite interesting.
| Quote: | | I would be interested to see you balance the equation for the reaction of Bi2O3 and NaOH to give NaBiO3. (OK, I know you meant to add the O2 as well,
but there are some less experienced folks who read this and would get puzzled) |
I wish I had a balanced equation, but I believe this is a non-stoichiometric reaction resulting in the formation of mixed oxidies and other products.
Personally I have never seen a balanced equation for a molten salt oxidation of any kind (with the exception of nitrates and the chlorate self
oxidation). I would be interested in any resources anyone knows of where I could read up on this however molten salt chemistry seems to have fallen
out of fashion.
| Quote: |
I don't see anything anywhere on trioxide/NaOH/air/no electricity giving the bismuthate, instead of heating with Na2O2, but my home library is
limited.
|
Due to my recent post about hydroxides being oxidizing agents and my research I can now make some explanation. I have seen that fusing Bi2O3 with
NaOH yeilds NaBiO3 but I cannot recall where. Recall though that the highly electropositive enviorment in molten hydroxides helps to stabilize high
oxidation states. Also there is an equilibrium in the melt:
2 OH- <----> H2O + O2-
That helps to contribute to the oxidation action of hydroxides, however this action alone is fairly weak and therefore the actual basicity of the
reaction medium is the driving force. I believe that the initial steps involve the oxidation of bismuth trioxide to bismuth pentoxide which reacts
with the melt in an acid-base reaction.
Bi2O5 + 2NaOH ---> 2NaBiO3 + H2O
But I am not sure on this as I didn't find anything to back this up 
So in summation, the hydroxide melt is basic enough/electron rich enough to stabilize high oxidation states and therefore help to overcome the energy
barrier to the oxidation by atmospheric oxygen. In addition considering the equilibrium existing in the melt it is possible that sodium peroxide
might be an intermediate and the reaction between straight sodium peroxide and bismuth trioxide to form sodium perbismuthate might just be a direct
way of doing this same reaction.
| Quote: | | BiCl3 when mixed with H2O will form BiOCl, but only if the excess of H2O/HCl is great |
My books on the subject state that BiCl3 solutions left standing slowly hydrolyze to BiOCl but did not state anything else, so almost immediately
after all the Bi had dissolved I precipiated to avoid bismuth oxychloride formation.
The preparation of dibismuth tetroxide from the Handbook of preparative inorganic chemistry gives this reaction:
"Potassium bismuthate is boiled for about 10 hours in a large excess of 10% perchloric acid, until a slight residue of an orange-red precipitate
is left. The percipitate is filtered off, washed and dried. This is hydrated Bi2O4."
2KBiO3 + 2HClO4 ----> 2KClO4 + Bi2O4 + O2
One of my chemistry books lists the use of bismuth tetroxide as part of a lubricant for dies, however I don't have anything on the bismuth 4+
cation and therefore dibismuth tetroxide might just be an oxide mix between bismuth tetroxide and bismuth trioxide, however I have no information
either way but it just appears superfically to be a valid compound.
[Edited on 6/1/2004 by BromicAcid]
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
So is there any way to dissolve Bi metal with salts of noble or semi-noble metals, such as CuCl2 or CuSO4? Similar to dissolving Pb with Cu(Acetate)2?
This would potentially provide a better route than using nitric acid (which I just hate to waste).
Alternatively, how about electrolyis?
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
More Mellor: "KBiO3 is also formed as a dark red deposit on the anode when an almost boiling solution of KOH (sp gr 1.43) and KCl in which Bi2O3
is suspended, is electrolysed in a platinum dish."
This oxide can be had as a crust by heating the metal to some point for some time.
Isn't H2O2/NaOH a rather strong oxidizer? Like Cl2? Eh?
|
|
|
ordenblitz
Hazard to Others
  
Posts: 259
Registered: 18-7-2004
Location: Northwest
Member Is Offline
Mood: Bohr'd
|
|
Today I attempted to make bismuth thiocyanate via the following:
Bi(NO3)3 + 3 KSCN --> Bi(SCN)3 + 3 KNO3
I have made Pb(SCN)2 before and decided to follow a similar method. Needing a soluble Bi salt, I checked Hawleys and Merck, pretty much by default,
Bi(NO3)3 was the choice.
I first made a solution of 2.91g of KSCN in 15ml water. Then 3.95 grams of Bi(NO3)3 in about 40ml water. According to Merck, bismuth nitrate is
soluble in H2O, however it really didn’t seem like any was going into solution. I have read that the nitrate is slowly decomposed in water to
subnitrate. I thought that I could mix the two solutions and form the thiocyanate before much decomposition happened. Being that the nitrate never
seemed to go into solution it is possible that the subnitrate formed fairly fast. Anyhow the water really didn't do much but turn yellow with the
nitrate/subnitrate? remaining unchanged and still pure white on the bottom.
Second try…
2.91g of KSCN in 10 ml water, warmed slightly to aid solution.
3.95 grams of Bi(NO3)3 was added to the above, in one proportion while stirring. The color changed immediately to a bright yellow orange. After a few
minutes the color deepened to orange red. At that point and I decided to check the ph, it was about 1.
I thought that this might be from residual acidity of the Bi(NO3)3, since it is supposed to smell like HNO3. Next I added KHCO3, in small proportions
since it was frothing nicely, to bring the PH to neutral. Whereupon the color vanished leaving a white powder that settled out of the water quite
nicely. I increased the solution to 50ml, filtered the solid and washed with several portions of hot water and is now drying.
The problem is that it looks suspiciouly like BiNO3(OH)2 * BiO(OH).
I think I might just have taken the long route to bismuth subnitrate?
I can find but one mention of Bi(SCN)3 while googling and it says nothing about its synthesis. Anyone have any ideas on a better route?
[Edited on 9-10-2004 by ordenblitz]
[Edited on 9-10-2004 by ordenblitz]
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
For the older part of the thread,
I would be more inclined to roast bismuth metal in air to make B2O3 rather than try to dissolve in acids. Its still my opinion that its the highly
basic conditions effecting the equilibrium and thus the redox potentials that enables weak oxidising agents to produce manganates, chromates,
bismuthates etc in the melt.
ordenblitz,
Ok, I'm going to make a stab at this. My main source of inorganic information is from Mellor, and he doesnt cover cyanides, cyanates or
thiocyanates.
Bismuth thiocyanate doesnt even apear in the CRC. So, some misc paper sources.
There seem to be 2 big gotchas, formation of basic salts of bismuth as you guessed and most specifically in the case of bismuth thiocyanate, formation
of bismuth thiocyanate complex anions. For example a mixture of hydrogen thiocyanate, potassium thiocyanate and bismuth carbonate produces
K3Bi(SCN)6.
The only prep I can find does have a reference attached, which is a bonus, but its not a simple metathesis.
"Bismuth carbonate is added to a 10% solution of hydrogen thiocyanate. Crystals of a yellow colour are formed. When a solution of hydrogen
thiocyanate is saturated with bismuth carbonate, and afterwards concentrated in vacuo over sulphuric acid, needles of bismuth thiocyanate are
formed."
One of these is possibly a basic salt as indicated by acompanying equations, but which is which isnt clear. The reference is,
A. Rosenheim and W. Vogelsang, Z. anaorg. Chem., 48, 205 (1906).
Hydogen thiocyanate seem to be rather difficult stuff to work with, if you just add acid to a salt it decomposes in the warm. Preperation here, the
only one I can find is,
"Hydrogen thiocyanate is prepared by the action of dilute sulphuric acid on alkali thiocyanates, with distillation under reduced pressure and
collecting distillate in reciever kept in a freezing mixture."
B.S. Sharma, J. Am. Chem. Soc., 52, 581 (1930).
Also a few older references. I cant get hold of any of the actual references but this might be enough information to experiment, particually if you
can find a more reliable method of making or getting HSCN. (Can it be stabilised enough to be sold?) I also have no idea of toxicity etc.
I'm curious, what is it you want bismuth thiocyanate for?
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
At a sufficiently high temperature, the nitrate may oxidize the thiocyanate. If Bi(SCN)3, or the basic salt BiO(SCN), could be made, it would be very
poisonous.
|
|
|
ordenblitz
Hazard to Others
  
Posts: 259
Registered: 18-7-2004
Location: Northwest
Member Is Offline
Mood: Bohr'd
|
|
Thanks for taking a stab at this one gents!
I have been looking for alternatives for Pb compounds used in electric matches. So far I have tested some interesting things but I am not satisfied
yet.
The most commonly used sensitive "bridge wire" compositions are; lead picrate, lead mononitroresorcinate and a mixture of PB(SCN)2 + KCLO3.
I have been researching bridgewire compounds for some time now. I've been working with Bismuth lately.
Today I attempted Bismuth picrate via TNP + Bi(NO3)3
But that didn't work so I will try sodium picramate tomorrow.
[Edited on 13-10-2004 by ordenblitz]
[Edited on 13-10-2004 by ordenblitz]
[Edited on 13-10-2004 by ordenblitz]
|
|
|
ordenblitz
Hazard to Others
  
Posts: 259
Registered: 18-7-2004
Location: Northwest
Member Is Offline
Mood: Bohr'd
|
|
Sodium picramate + Bi(NO3)3 does work but the yield is low.
The bismuth picrate I made is interesting physcally. It produces very small crystals that are somewhat plastic. They are soluble in acetone and water
but not alcohol. In small amounts it burns with a quiet thump and is surprisingly clean.
[Edited on 15-10-2004 by ordenblitz]
|
|
|
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
An attempt at potassium bismuthate

3 grams of bismuth hydroxide or a similar material (i.e., the product of adding KOH solution to a solution of Bi(NO<sub>3</sub> <sub>3</sub> <sub>3</sub> was placed into a steel crucible and heated for several minutes over a propane torch. Steam was released and the color of the material
lightened to almost white, though it was a little difficult to tell since there was some carbon powder left in the crucible from a previous
experiment. After I was confident it was dehydrated I added a few pellets of solid KOH. They didn't do much or dissolve until suddenly they fizzed
and popped and melted very rapidly, possibly exothermically (a reaction with the bismuth materials in the crucible?) Within minutes the suspension of
material in the liquid KOH had turned green/yellow and the solution itself looked green. I let this continue for an hour stirring from time to time
with a nickel rod (the liquid KOH was green before I started stirring it with the nickel rod). was placed into a steel crucible and heated for several minutes over a propane torch. Steam was released and the color of the material
lightened to almost white, though it was a little difficult to tell since there was some carbon powder left in the crucible from a previous
experiment. After I was confident it was dehydrated I added a few pellets of solid KOH. They didn't do much or dissolve until suddenly they fizzed
and popped and melted very rapidly, possibly exothermically (a reaction with the bismuth materials in the crucible?) Within minutes the suspension of
material in the liquid KOH had turned green/yellow and the solution itself looked green. I let this continue for an hour stirring from time to time
with a nickel rod (the liquid KOH was green before I started stirring it with the nickel rod).
Finally the liquid was poured out on a steel plate, the upper most 'slag' was blue tinted and the material more toward the bottom was green/brown. A
few pieces of this were tossed in some water and dissolved, the solution turned brown from suspended bismuth hydroxide or whatever particulate, but at
the very bottom of the test tube a yellow layer settled (pointed out with the arrow above). The solution bubbled from time to time but I did not do
any tests to determine if this was indeed my potassium bismuthate. However I did retain all of the KOH/Bismuthate solid that I poured out molten and
will do some tests on it later. Also the test tube pictured above is still setting in my shed and the bismuthate should decompose from being in water
so I will be able to tell if the yellow material decomposes.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Bismuthates, along with plumbates, are very powerful oxidizing agents, able to oxidize Mn(II) to permanganate and Fe(II) to ferrate(VI) in alkaline
solution. This is, in fact, the basis of a colorimetric/spectrophotometric method for the determination of Mn in aqueous solution in the absence of Fe
(which has a similar color and so interferes). The only way they can be prepared pure, and free of Bi(III), is by electrolysis of a cold alkaline
solution of Bi(III).
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
There does not seem to be much info on bismuthate in the books, they don't even agree on the reduction product of bismuthate. I have seen Bi2O3, BiO+
and Bi3+ as the reduction product. This may be just due to the ammount of water present however, as 3+ bismuth tends to hydrolyse quite easily.
I tried the oxidation of Mn2+ with bismuthate acidified with nitric acid(Don't use HCl ...oops), a few problems with it as an oxidizing agent are apparent, first its insolubility in water, and secondly its very high molar
mass, it took over 4.5g of bismuthate(91.8%, technical) to oxidize 1g Mn(OAc)2 to permanganate. Would take quite a bit for most oxidations. I added
some sodium carbonate to the bismuth containing insoluble reduction product, hoping for bismuth subcarbonate, which should help determine whether
Bi2O3 or BiO+ or Bi3+(likely just as intermediate) is formed(I hope). ...oops), a few problems with it as an oxidizing agent are apparent, first its insolubility in water, and secondly its very high molar
mass, it took over 4.5g of bismuthate(91.8%, technical) to oxidize 1g Mn(OAc)2 to permanganate. Would take quite a bit for most oxidations. I added
some sodium carbonate to the bismuth containing insoluble reduction product, hoping for bismuth subcarbonate, which should help determine whether
Bi2O3 or BiO+ or Bi3+(likely just as intermediate) is formed(I hope).
[Edited on 27-7-2006 by rogue chemist]
|
|
|
Axt
National Hazard
   
Posts: 814
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
I had a try at sodium bismuthate. No real conclusions because I'm not real sure what this chocolate coloured compound is supposed to be.
Bismuth nitrate: 50g bismuth was dissolved in 100ml 70% nitric acid, the reaction with crushed bismuth is rapid and a copious quantity of NO2 is
released leaving a pale green solution.
Bi + 6HNO<sub>3</sub> --> Bi(NO<sub>3</sub> <sub>3</sub> + 3NO<sub>2</sub> + 3H<sub>2</sub>O <sub>3</sub> + 3NO<sub>2</sub> + 3H<sub>2</sub>O
Bismuthyl hydroxide: 50ml water was added to the solution, and then 20% sodium hydroxide was dropped in until the solution formed a permanent
precipitate. A further 30g sodium hydroxide in 50ml water was then added forming a large precipitate of bismuthyl hydroxide. The solution was heated
to 100°C for 30 minutes then poured into 1L of water, stirred and filtered.
BiO(OH) + NaOBr --> NaBiO<sub>3</sub> + NaBr + H<sub>2</sub>O
Bromine: Forget the actual quantities used, but it followed the following stoichiometry to provide 1.5x excess, with enough water so there was no
precipitate of sodium bisulphate.
2NaBr + 2H<sub>2</sub>SO<sub>4</sub> + H<sub>2</sub>O<sub>2</sub> --> Br<sub>2</sub> +
2NaHSO<sub>4</sub> + H<sub>2</sub>O
Sodium bismuthate: : The filter cake of bismuthyl hydroxide was mixed into a suspension with 150g sodium hydroxide and 150ml water. The suspension was
then heated to 130°C and the bromine water solution added slowly with rapid stirring over the course of 30 minutes.
2BiO(OH) + 6NaOH + 2Br<sub>2</sub>--> 2NaBiO<sub>3</sub> + 4NaBr + 4H<sub>2</sub>O
Now that gives the chocolate brown precipitate as mentioned in Brauer, however when washed with water it doesn't turn yellow.
"The suspension is now agitated for a while, until the color changes from brown through light brown to yellow." Brauer pg. 628.
After a number of washes, still no go. I gave up at this point and just left it sit under water, now clear crystals have grown out of the brown
compound. Anyone hazard an explanation?
Top left is suspension of BiO(OH) in 50% NaOH and bromine solution next to it. Top right is after adding the Br2. Bottom is crystals growing on the
"brown stuff".

|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
Can anyone out there help me to achieve Bismuth Trichloride.
I attempted to put Bismuth into HCl as an Anode but just got some black deposit on the Cathode after a short space of time. I presume it is spongy
metallic Bi.
HCl + H2O2 dosent work.
The only way I can see (for the garage dude) is Aqua Reiga where I read (KO) that it will dissolve Bismuth and you get a hydrated form of BiCl3 that
you can distill if you want the anhydrous form.
The thread above suggests adding Bi Hydroxide to HCl to get BiCl3. Will this work?
I have to make Bi Hydroxide first obvioulsy. Post above (at the top) states that adding KOH solution to a solution of Bismuth Nitrate gives the
(insoluble) Bismuth Hydroxide? Is that bull? It's surly the Subnitrate or somesuch that is be precipitated, not the Hydroxide. Kirk-Othmer states that
the Hydroxide does not exist. (just to add some more confustion)(EDIT, opps not exactly, it stated the Hydroxide has not been isolated!)
I have Bi Oxide and Bi metal. Bi Nitrate is easy to make by just adding Nitric acid to Bi Metal.
When I add the Bi Nitrate to water I get a white precipatate as it hydrolized to something (the subnitrate).
But when I add the Bi Nitrate to distilled methylated spirits (no water) I get a white precipitate as well? What is that?
US Pat 4272354 gives a procedure that requires Chlorine gas (dry) going into molten Bi. That's a job I don't fancy.
Wiki states that you can make BiCl3 using Bi metal + HCl gas. That's bull AFAICS.
Royal Water + Bi metal it's gonna have to be....... I think?
Your's in ignorance,
Dann2
I enclose for your pleasure some Kirk-Othmer blurb on Bismuth.
Attachment: KOBismuth.pdf (211kB)
This file has been downloaded 1904 times
[Edited on 6-10-2010 by dann2]
|
|
|
Trifluoroacetic
Hazard to Others
  
Posts: 128
Registered: 6-8-2008
Member Is Offline
Mood: No Mood
|
|
I know this is slightly off track but while searching for bismuth thiocyanate I found the following:
Semiconducting properties of silver thiocyanate
The authors report measurements of the band gap of AgCNS by the study of optical absorption. It is concluded that silver thiocyanate is a p-type
semiconductor with band gap approximately=3.4 eV
K Tennakone and R H Wijayanayake
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
This should be helpful information
Glycerin or ethylene glycol may possibly function similarly to produce a soluble complex with bismuth nitrate which may facilitate manufacture of
other compounds from the soluble intermediate. It would be interesting to see what the nitrite and/or the nitrite plus HCl soluble product does when
added to ammonium hydroxide, as well as what occurs when added to ammonium carbonate or ammonium bicarbonate solution.
One or more of those reaction scenarios would probably lead to an ammonium bismuthate solution. Acidification of an ammonium bismuthate solution
could possibly lead to precipitation of a hydrated "soft" (reactive) hydroxide, which could be separated and reacted with organic acids, acetic,
tartaric, citric, methanesulfonic, ect. The possibility exists also for soluble double salts of the bismuth and the ammonium organic acid salts.

[Edited on 5-11-2010 by Rosco Bodine]
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
I doubt Bromic is still interested in a 6 year old thread but I just found the book I lost years ago. I had planned on scanning the Bi info to post
here. I guess better late than never.
     
I wanted to post the bigger scans to show the subscript numbers more clearly but the board will not allow them.
[Edited on 11-10-2010 by IrC]
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
It was dann2 who was asking about how to make bismuth trichloride which inspired my oblique response suggesting different soluble bismuth salts which
are thermodecomposible to the oxide at moderate temperatures, knowing that dann2 is working on anode coating precursor sols. Bismuth trichloride will
first hydrolyze
to bismuth oxychloride which will remain unchanged even at red heat, therfore it is certain that bismuth trichloride would need to be converted to an
alcoholate or some other thermodecomposible precursor. Bismuth Nitrate is a convenient precursor for Bismuth Trichloride, via Bismuth Oxychloride
which is gotten as the insoluble precipitate when a strong solution of KCl or perhaps NaCl is added to
a dilute HNO3 solution of Bi(NO3)3. The insoluble Bismuth Oxychloride is filtered
and rinsed, and is converted to Bismuth Trichloride by treatment with HCl.
Bismuth Trichloride is only stable in excess HCl and hydrolyzes back to insoluble
Bismuth Oxychloride when the pH is not held sufficiently low by an excess of HCl.
Bismuth Nitrate is similarly easily hydrolyzed to an insoluble basic nitrate if the
pH is not held sufficiently low by an excess of HNO3, but an advantage for Bismuth Nitrate is that hydroytically stable soluble complexes are formed
with polyols, and such complexes provide a convenient form of "soluble bismuth"
from which other salts can be readily made. As a bonus the polyol complexes
of Bismuth Nitrate are thermodecomposible.
Bismuth Ammonium Citrate and the polyol complexes of Bismuth Nitrate and
Ammonium Bismuthate Sol are a few "soluble bismuth" systems which I have found that are hydrolytically stable under mild pH conditions, also being
readily thermodecomposible to the oxide. It would be surprising if lower alcoholates of Bismuth have stability as good as the polyol complexes, or
the other two compounds. It would be unexpected for lower alcoholates to have a lesser stability even temporarily good enough for use as a coating
precursor for baked anode applications.
The most intriguing of the inorganic soluble bismuth salts reported is the insoluble nitrite subsequently treated with HCl to form a soluble product
which does not precipitate on dilution. If what is reported is true, this would be anomalous hydrolytic stability for a soluble inorganic bismuth
salt. However, the thermodecomposition may not be favorable if it leads to a bismuth oxychloride rather than the oxide. Evenso, the bismuth nitrite
- hydrochloride could still possibly be useful as a soluble intermediate for other
bismuth salts, if indeed it does not preferentially simply decompose to bismuth oxychloride in whatever further reactions are attempted.
[Edited on 10-11-2010 by Rosco Bodine]
|
|
|
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
IrC,
Always interested in a 6 year old thread! Thanks for the scans.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
A possibly related note, when some researchers were working on Ti complexes for use in/with paints and other coating applications, di- and tri-
ethanolamines gave reasonably stable water solubile or disperable complexes that would break down when backed at 80 to 200 C (depending on exact
complexing). The nitrogen of the amine complexed with the Ti, which was bound to the 2 or 3 oxygens as alkoxide; various other groups went on the 4th
Ti valency.
Citrate based systems would seem to be readily accessible to amateurs, fairly easy to experiment with BiCl3 and citrate salts in alcoholic solutions
with the goal of getting citrate complexes in solution.
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
You are welcome Bromic. I thought about posting these pages the day you started this thread but could never find the book. The 3rd page is scanned
badly so below is a redo. I cannot tell even holding the book what the missing part of the page is (end of first 3 or 4 Bi entries on p 563).
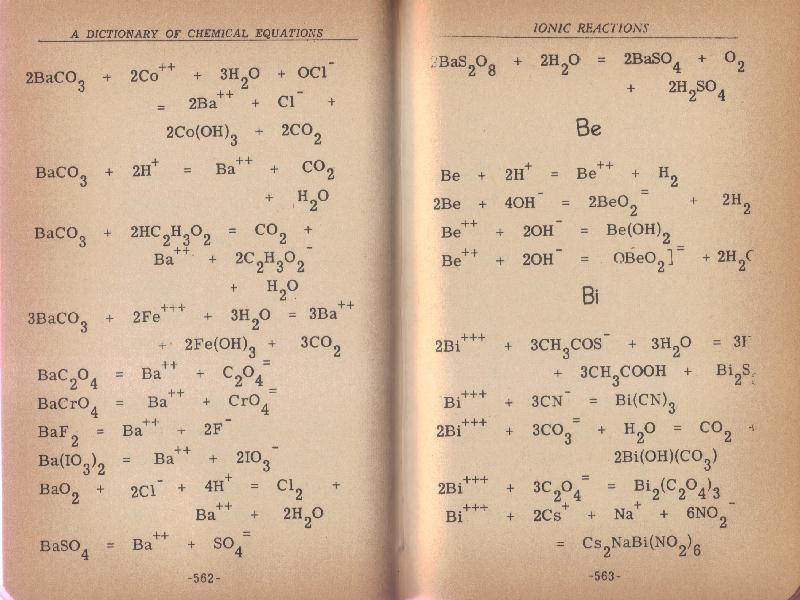
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
| Pages:
1
2 |