| Pages:
1
2
3 |
greenimp
Harmless

Posts: 42
Registered: 20-12-2008
Member Is Offline
Mood: No Mood
|
|
i always like the HCL into H2SO4 w/good stirring for gas generation. Something about liquid-liquid mixing makes for a more even gas flow....
Neon Sign place might be the key, but why not a big piece of stainless from swag lock? Then you could just use swag fittings at all the connection
points. That would help on the escaping gas problem, and make the whole process safer. Also, keep in mind here the goal is to come up with something
the any person can build in their back yard and product 100's of grams of AlCL3. IMO it needs to be bullet proof.
As to the yellow color, all the AlCl3 I used for my experiments was always slightly yellow. Didn't hurt anything as far as I could tell. Al foil
should be the target for the substrate. Getting Al powder could be come difficult some day. Foil fill always be out there. I think my not so dry
DCM was the bigger problem.
Also why not try some kind of cold finger for the condenser? Seems they were invented for this kind of work. You might need a really mean one, but it
would be the best IMO. They are not to hard to build out of a mason jar and copper tubing.
Green Imp
|
|
|
spong
Hazard to Others
  
Posts: 128
Registered: 28-5-2009
Location: Chatham
Member Is Offline
Mood: No Mood
|
|
Ooh thanks  It would have been about 2AM, left a maths assignment to the last
minute It would have been about 2AM, left a maths assignment to the last
minute 
Yeah the powder would probably react too quickly anyway, plus it would want to clump at the bottom of the tube on the glass, foil worked well because
it wouldn't be contacting the glass all that much and wouldn't transfer much heat to it as it burnt. Stainless would be good but I don't have the
money for it, plus I like being able to see what's going on in there. The cold finger would be better than cramming powder from a tube periodically,
you could keep a spare lid so once it's over you just jiggle the finger from outside the lid to shake the last of the powder off, then remove the jar
and put a new lid on it. You could probably leave the one jar on for the whole reaction and just disconnect it when it's done and keep filling it the
next time you do it. I might try that fir the next run if I can get a nice smooth bend in the glass from the reactor tube, like this:
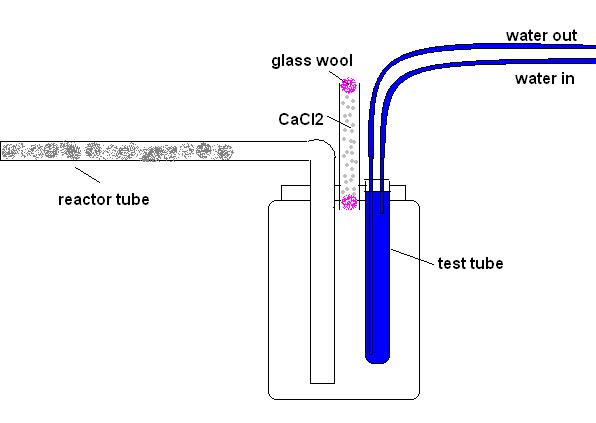
The only thing I'd be worried about is the reactor tube clogging before it ends in the jar, the stuff sublimes on anything that isn't insanely hot so
a lot would probably collect in the tube.
[Edited on 30-8-2010 by spong]
|
|
|
greenimp
Harmless

Posts: 42
Registered: 20-12-2008
Member Is Offline
Mood: No Mood
|
|
jacketing the tube as it leaves the BBQ and pumping warm air or hot motor oil through the jacket could work to keep things hot till the condenser.
Wide tubeing is probably key also. 1 inch would be a good starting point. Thing is you guys have it working. I cant wait to fire up the grill now,
regardless of the setup.
Green Imp
|
|
|
Chainhit222
Hazard to Others
  
Posts: 138
Registered: 22-8-2009
Location: peach's mailbox
Member Is Offline
Mood: grignard failing to start
|
|
I have started the era of BBQ chemistry.
If you use a condenser jacket with hot motor oil, how could you heat and recirculate the oil? Would a pump hold up to those temps? Or do you just
intend on using the oil to hold temperature?
This is certainly interesting. I would love to see a more complicated setup.
[Edited on 1-9-2010 by Chainhit222]
The practice of storing bottles of milk or beer in laboratory refrigerators is to be strongly condemned encouraged
-Vogels Textbook of Practical Organic Chemistry
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Chainhit222  | | If you use a condenser jacket with hot motor oil, how could you heat and recirculate the oil? Would a pump hold up to those temps?
|
I picked up a little gear pump last year at surplus. I found out after I got it home that it was a pump for
recirculating hot frying oil, so it's pretty much exactly what you want for this. (You can't have mine, no no no.) Gear pumps are also used for
hydraulic systems. Scavenging one from a repair shop might be possible, say, if its internal seals are failing but not totally failed. A pump that
doesn't quite maintain hydraulic pressures would be just fine for recirculating oil.
P.S. Here's an example of a gear pump from Grainger. It's the bottom of series all of which are about $150 new. Discount accordingly for surplus and scavenge.
[Edited on 1-9-2010 by watson.fawkes]
|
|
|
greenimp
Harmless

Posts: 42
Registered: 20-12-2008
Member Is Offline
Mood: No Mood
|
|
I have built the set up last night waiting for the weekend to run. Using 1/2 inch stainless, the tubes are 1.5 feet long and have quick disconnects
to swap the out. Working on a cold finger tonight. I hope to make 500 grams this weekend. Cross your fingers...if it works ill put the part list up.
Green Imp
|
|
|
Chainhit222
Hazard to Others
  
Posts: 138
Registered: 22-8-2009
Location: peach's mailbox
Member Is Offline
Mood: grignard failing to start
|
|
Quote: Originally posted by greenimp  | I have built the set up last night waiting for the weekend to run. Using 1/2 inch stainless, the tubes are 1.5 feet long and have quick disconnects
to swap the out. Working on a cold finger tonight. I hope to make 500 grams this weekend. Cross your fingers...if it works ill put the part list up.
|
fucking outstanding 
I just hope your stainless does not react... are you using Cl2 or HCl?
[Edited on 2-9-2010 by Chainhit222]
The practice of storing bottles of milk or beer in laboratory refrigerators is to be strongly condemned encouraged
-Vogels Textbook of Practical Organic Chemistry
|
|
|
densest
Hazard to Others
  
Posts: 359
Registered: 1-10-2005
Location: in the lehr
Member Is Offline
Mood: slowly warming to strain point
|
|
Stainless + Cl- + H2O -> no more stainless, unless it's a chloride-resistant alloy!!!
Seriously, chloride/chlorine eats stainless for breakfast and comes back for more. That's why it isn't generally used in marine applications.
If everything is ----perfectly---- dry, it might work.
[Edited on 2-9-2010 by densest]
|
|
|
spong
Hazard to Others
  
Posts: 128
Registered: 28-5-2009
Location: Chatham
Member Is Offline
Mood: No Mood
|
|
Ohh I can't wait to see the results, are you going to use HCl or Cl2? With the comments above I'd hope HCl, if it doesn't react with copper then it
should be fine. Have a blowtorch handy to heat the section of tube between the BBQ and condenser so it doesn't clog, that's another advantage of
steel, you could blast it with the torch and not have it shatter.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
I didn't want to throw too much negative nancy-ism around all the positive vibes, but densest is suggesting something I was worried about as well.
Equipment for handling things like hydrogen chloride is usually made of weird, expensive alloys like Monel. I can get normal regulators for tens of
pounds. Reactive gas regulators are £500 or so.
| Quote: | | Compared to steel, Monel is very difficult to machine as it work-hardens very quickly. It needs to be turned and worked at slow speeds and low feed
rates. It is resistant to corrosion and acids, and some alloys can withstand a fire in pure oxygen. It is commonly used in applications with highly
corrosive conditions. Small additions of aluminium and titanium form an alloy (K-500) with the same corrosion resistance but with much greater
strength due to gamma prime formation on aging. Monel is typically much more expensive than stainless steel. |
| Quote: | | Monel is a trademark of Special Metals Corporation for a series of nickel alloys, primarily composed of nickel (up to 67%) and copper, with some iron
and other trace elements. |
This isn't just industrial, commercial theory either, it's everyday practical reality unfortunately.
Stainless steel scissors

This sink, despite it being old and retired from the kitchen, was clean prior to me using hydrogen chloride near it

The brand new chromed tap is turning green, it doesn't show up very well here, but you can see the stainless is rotting

The thing for doing the cleaning up needs cleaning up

The shiny new regulator has tarnished and features a new, green, colour scheme, despite being metres away

This is all from one or two minute escapes of the bastard. In todays politically correct atmosphere, hydrogen chloride is a bigoted & intolerant
character.
It'll probably work, at least for while. However, I would expect some degree of contamination and for the corrosion to occur a lot quicker when the
gas is hot.
You may be able to slow the rate down by ensuring the gas is well cleaned up from the generator. That means getting it bone dry and getting the
sulphuric out, which will start ruining your Lewis acid anyway.
[Edited on 2-9-2010 by peach]
|
|
|
greenimp
Harmless

Posts: 42
Registered: 20-12-2008
Member Is Offline
Mood: No Mood
|
|
My setup will be hcl/h2so4 for HCL generation w/ nitrogen or He carrier gas. If will also use the carrier gas to purge the system before and after
running. As long as i keep everything dry ss should be fine. Even if I only get two or three runs, it is a good place to start. I also have a motor
oil pump that I will use to jacket the line from the bbq to the cold finger. If it clogs I will be very surprised. I'm in about 200$ on this but I
have all the glassware and most of the fittings already. I am looking on CL for a used grill, don't want to use my new one.... Can't believe I am
going to work with hcl gas again I vowed never again after my last round of exps.....
What do you guys think for drying? Bubbleing through a talk column of h2so4 plus a drying tube?
Green Imp
|
|
|
spong
Hazard to Others
  
Posts: 128
Registered: 28-5-2009
Location: Chatham
Member Is Offline
Mood: No Mood
|
|
Yeah CaCl2 and H2SO4 should work fine, are you going to add a tube of activated carbon too? I'll be getting some glass tubes on Wednesday so next
weekend I'll have another shot.
|
|
|
spong
Hazard to Others
  
Posts: 128
Registered: 28-5-2009
Location: Chatham
Member Is Offline
Mood: No Mood
|
|
I had another shot with tubes from a neon light supplier, 25mm glass. It wasn't a huge advantage to the fluro light tube I was using but at least it's
a bit sturdier and I'm not being exposed to mercury cleaning them. I didn't use the activated carbon but it still worked ok.
One thing I noticed with a clear condenser was the AlCl3 seemed to just settle as a powder at the bottom of the condenser, to empty it all I had to do
was pull it out and give it a slight tap above the jar, much easier than ramming a cork down there. I added it to the small amount I obtained from the
last reaction.
here's the first AlCl3 subliming:

The powder settling in the bottom of the tube:

The jar of AlCl3, one day I may fill it up to the top 

|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Nice work, Spong! Do you have any method for verifying its purity or potency? Does it hiss when placed on water?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Do you have activated carbon spong? I can send you out a little scoop to fill a test tube if not, since you're actually doing as
opposed to just contemplating. The price tag will be some photos of the setup and result. 
I'd be careful with the hissing test. I pulled a little plug of white gunk out of my DCM AlCl3 method, threw it in some water and it hissed. But I
wouldn't accept that as proof that it was working.
If it doesn't smell of anything, or much, at first, and then stinks of hydrogen chloride whilst hissing, you're onto a winner. Holding a bit of damp
acid indicating paper in the fumes will show it up for MOAR evidence.
|
|
|
aonomus
Hazard to Others
  
Posts: 361
Registered: 18-10-2009
Location: Toronto, Canada
Member Is Offline
Mood: Refluxing
|
|
I'm left wondering whether aluminum turnings from a lathe or sheet aluminum from the hardware store would fare any better in terms of purity. I don't
really have the equipment to do this (or the privacy), but perhaps one of you guys could give this try to avoid iron impurities? Most aluminum alloys
are fairly well characterized so you'd be able to know what other impurities may form in HCl given their composition.
|
|
|
spong
Hazard to Others
  
Posts: 128
Registered: 28-5-2009
Location: Chatham
Member Is Offline
Mood: No Mood
|
|
It definitely fizzes on contact with water  It really heats up
a lot too. The fumes are definitely acidic, I had a big solid lump of it left in a flask after a vacuum sublimation of it today and I was playing
around with it by adding water and watching it fizz and pouring the dense HCl 'smoke' into flasks and stuff and then I plugged the flask and shook it
around to dissolve the HCl into the water in there and dumped it out on the driveway, it fizzed with some carbonates in the gravel we have and a worm
that was on the ground wiggled like crazy. Poor thing, I picked it up and rinsed it off but it was dead It really heats up
a lot too. The fumes are definitely acidic, I had a big solid lump of it left in a flask after a vacuum sublimation of it today and I was playing
around with it by adding water and watching it fizz and pouring the dense HCl 'smoke' into flasks and stuff and then I plugged the flask and shook it
around to dissolve the HCl into the water in there and dumped it out on the driveway, it fizzed with some carbonates in the gravel we have and a worm
that was on the ground wiggled like crazy. Poor thing, I picked it up and rinsed it off but it was dead 
I don't have any carbon but I can get some from a pet shop for filters so no need to ship any  I will get photos when I do it though I will get photos when I do it though 
Anyway, here's the pictures from today:
The setup(I need to buy a cold finger next time I get some glassware):

The dirty AlCl3

Cooling and tipi:

Just starting:

Finished:

The crud that was left behind in the flask (a lot of it was AlCl3 as I discovered when I went to clean it  ) )

All I could recover from the receiving flask:

Al turnings would probably be better, I need a good source of them, is there an easy way to make large amounts from a block of Al?
[Edited on 10-9-2010 by spong]
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
That's absolutely great! We need more pictures and videos and people getting hands on.
I like the stopper in that flask, smooth.
A cold finger would likely be better, but that looks quite good already. Try adding ice / gel packs to the beaker in the meantime.
That vivid yellow has got to be iron chloride.
For turnings, open the yellow pages and call all the guys offering machining, even the guys resurfacing engines may be able to help (Ferrari engine
blocks are magnesium, if you can find anyone servicing those  ), the scrap
dealers will know where to get it as well. The CNC mills and lathes SPEW Al turnings off so quickly they have big augers or conveyors
under the table to push all the stuff out into a huge bin on wheels next to the machining centre. ), the scrap
dealers will know where to get it as well. The CNC mills and lathes SPEW Al turnings off so quickly they have big augers or conveyors
under the table to push all the stuff out into a huge bin on wheels next to the machining centre.
It all goes back to the foundry as comparably high purity precursor to be recast; compared to the ore it's self. But, the machine tools almost always
drench the chips and turnings in coolant, which is basically oil in suspension. That lowers it's value a little because the foundry has to then deal
with all the smoke and shit burning back off, and clean the alloying up.
Some of the CNC places produce so much of it, they have autobailers that empty the bins into a hydraulic press to squash it into a smaller brick they
can stack up as they wait for the scrap guys to turn up, so it's not using up expensive floor space. I've seen some of those bailers with automatic
washers on them to rinse the material of coolant prior to squishy the metal into a brick.
I have two billets of aluminium about two foot long each, which I bought from a foundry supplier in Wales (home of very expensive cartons of milk).
They'll be pure, but reducing them to shavings, turnings or chips isn't all that easy. Best to go with the scrap I expect.
The most amusing video on youtube of aluminium under pressure
^^^There are also some very funny ones of gigantic 1000 ton hydraulic hammer forges smacking bits of red hot iron into shape
|
|
|
aonomus
Hazard to Others
  
Posts: 361
Registered: 18-10-2009
Location: Toronto, Canada
Member Is Offline
Mood: Refluxing
|
|
If you do get your hands on a bucket of Al turnings/chips, probably a few successive extractions with something like acetone will get rid of all the
organics that might get turned into the typical black or brown tar on contact with gaseous HCl. Just use solvent grade stuff and redistill the whole
lot, its not worth using something *really* nice.
|
|
|
spong
Hazard to Others
  
Posts: 128
Registered: 28-5-2009
Location: Chatham
Member Is Offline
Mood: No Mood
|
|
Ohh yeah I could have a hunt around machining places, someone would definitely have some to spare. Magnesium would be great  Copper would be handy as well. Copper would be handy as well.
Yeah I'd have to wash the oil and whatnot off, I'd definitely have to distill the acetone back with the prices these days, $10 a litre :/
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
It's those tarts making bombs hoarding all the acetone
|
|
|
MolecularConcept
Harmless

Posts: 3
Registered: 10-9-2010
Member Is Offline
Mood: No Mood
|
|
how about using a chromatography column for the reactor tube? its thick pyrex and has correct fittings.
great work guys so is it practical for making 100s of gramms yet ?
|
|
|
spong
Hazard to Others
  
Posts: 128
Registered: 28-5-2009
Location: Chatham
Member Is Offline
Mood: No Mood
|
|
I think it's bunnings having their fun with the monopoly everything is expensive, they wanted $100 to order in 4l of toluene!
With turnings it should be much easier to make large amounts, I've just bought a big bottle of hypochlorite and a bag of charcoal so once I get some
Al turnings I can have a try at making hundreds of grams, I think the oxide 'ash' the foil leaves behind is preventing the centers of the bunches from
reacting, turnings should have a much lower oxide to Al ratio and I could cram hundreds of grams of turnings in the tube.
The new reactor tube will just have a small tube for the hose stuck at one end and the open end for the condenser at the other, very similar to a
chromatography column without the stopcock so they should be pretty good if you can find a really long one. It won't be possible to reload it easily
while it's running because the oxide would have to be washed out from the small end but with the amounts of Al in there something else will surely go
wrong before I run out of Al.
Now to ring around and find some turnings 
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
$10 a liter??! Christ! And I thought it was expensive here (~$5 a quart which is about a liter).
It's also much cheaper to buy it in 5 gallon containers at a solvent supplier but what to do with the unused portion?
Also, toluene is widely available here. $15 or so for a gallon (~4 L) and about $45 for 5 gallons.
What's up with the lack of acetone, toluene and various other petrochemicals in other countries (outside U.S.)?
[Edited on 9-20-2010 by MagicJigPipe]
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
spong
Hazard to Others
  
Posts: 128
Registered: 28-5-2009
Location: Chatham
Member Is Offline
Mood: No Mood
|
|
Yeah I've already bought 20l of toluene and methanol because that's the only reasonable way to get them. The shed is a big enough fire hazard without
another 20L of flammable liquids  Getting acetone from a chemical supplier is
actually cheaper than from the hardware store *facepalm* Getting acetone from a chemical supplier is
actually cheaper than from the hardware store *facepalm*
I think it's mainly Australia. Someone at some point has decided the general public has no use for paint thinners.
|
|
|
| Pages:
1
2
3 |