Mildronate
Hazard to Others
  
Posts: 428
Registered: 12-9-2009
Member Is Offline
Mood: Ruido sintetico
|
|
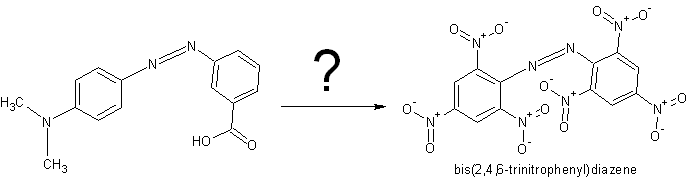
bis(2,4,6-trinitrophenyl)diazene from methyl red
Is it possible to make bis(2,4,6-trinitrophenyl)diazene from methyl red or some else explosive from it?
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
Doubt it. Getting that carboxylic acid and dimethylamine off would be a pain in the ass, and I doubt it could be done without destroying the azo
linkage anyway. Just nitrate aniline to trinitroaniline and then form the azo compound from that.. Much easier I suspect.
|
|
|
Mildronate
Hazard to Others
  
Posts: 428
Registered: 12-9-2009
Member Is Offline
Mood: Ruido sintetico
|
|
i had many botlles of methyl red. Maybe is possible this way?
[Edited on 18-9-2010 by Mildronate]
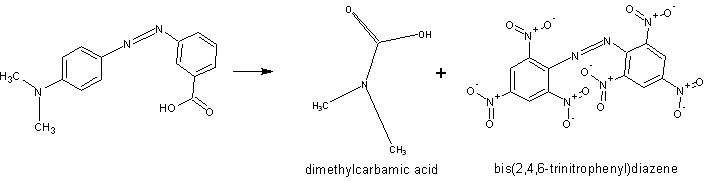
[Edited on 18-9-2010 by Mildronate]
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
Any references for that? I really doubt those C-C and N-C bonds are going to break the way you want.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Bis(2-4-6 trinitrophenyl)diazene (aka hexanitroazobenzene (HNAB)) is prepared by reacting dinitrochlorobenzene and hydrazine and nitrating and
oxidising the tetranitrohydrazo product by mixed acid. . .
You can forget methyl red!
|
|
|
Mildronate
Hazard to Others
  
Posts: 428
Registered: 12-9-2009
Member Is Offline
Mood: Ruido sintetico
|
|
Why forget structure is very similar.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Why waste time grasping at straws - as 497 said, removing the side-chains while keeping the nucleus intact would probably be quite a feat. . .
And nitrating azobenzene itself is likely to be anything but straightforward!
|
|
|