| Pages:
1
2 |
FLchem10
Harmless

Posts: 20
Registered: 29-7-2010
Member Is Offline
Mood: No Mood
|
|
Questions about PICRIC?....
Can Picric be mixed with plasticizers such as nitrocellulose or some other type...
I know it isnt usually used like that but, Is it possible and what type would be best?
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
With few exceptions a plasticizer is nearly always inert , being present
in a composition solely for providing an explosive as a dough. Although
Nitrocellulose combined with liquid organic nitrate compounds forms a
few semiplastic compositions , this does not make it a plasticizer.
I know of no mixture with Trinitrophenol intended to make it moldable
for expedient application in the field. The likely reason is that it requires
confinement to detonate properly so any inert additive will only make it
less so.
Unlike organic nitrate compounds which only produce a headache from
handling , Most nitro compounds , Picric acid in particular are toxic and
should not be handled. Another reason there are no plastic nitro
compounds that come to mind.
.
|
|
|
FLchem10
Harmless

Posts: 20
Registered: 29-7-2010
Member Is Offline
Mood: No Mood
|
|
I had figured that was what anyone who replied would say thanks....
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by FLchem10  | Can Picric be mixed with plasticizers such as nitrocellulose or some other type...
I know it isnt usually used like that but, Is it possible and what type would be best? |
I was perusing Henry Drinker's 1883, A Treatise of Explosive
Compounds and Rock Drill and Blasting last night and happened
upon this.
Max Tschirner's 1880, US Patent 232 381. Explosive Compound.
57 parts of picric acid and 43 parts of chlorate of potash......well
incorporated together , with the addition of 5 per cent
of pulverized rosin. I then sprinkle the product with a sufficient
quantity of benzine, kerosene-oil, or other fluid to moisten it,
which will readily dissolve the rosin and.... the compound
becomes a a plastic mass easily molded.
{Yeabut ... what happens when the solvent evaporates....}
djh
----
Science is a collection
of successful recipes.
Paul Valéry
French poet-essayist.
(1871-1945)
|
|
|
pjig
Hazard to Others
  
Posts: 179
Registered: 25-5-2010
Member Is Offline
Mood: always learning
|
|
If you have access to nitro methane, there is a patent that used a mix of picric acid and nitro methane that is claimed to be very powerful. Im
assuming that you might be able to mix potassium chlorate to thicken up mass to a dough like consistency.
|
|
|
Jimbo Jones
Hazard to Others
  
Posts: 102
Registered: 15-10-2009
Member Is Offline
Mood: No Mood
|
|
Picric acid + potassium chlorate = very bad idea.....
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
By itself Nitromethane is much harder to detonate than Trinitrophenol. Interestingly
Ammonium Nitrate most insensitive of all , added to Nitromethane forms a shock
sensitized mixture commercially known as Kinepak. Very slightly more than one part
Nitromethane ( 122 grams ) to two parts of Ammonium Nitrate ( 240 grams ) is
stoichiometrically balanced for oxygen. 2 CH3NO2 + 3 NH4NO3 => 2 CO2 + 9 H2O + 4 N2
Because Nitromethane is very much more expensive than Ammonium Nitrate , composition
can be as low as 16 parts Ntromethane to 84 parts of Ammonium Ntrate , with reduced
power. These pasty blends are not suitable for storage and only made for immediate use.
Up to 7 Parts of Trinitrophenol are soluble in one part of Nitromethane , remaining liquid
at room temperature. Perhaps including up to two additional parts of Ammonium Nitrate
may make a semiplastic material , I don't know , and stability in storage is a concern.
Amatol made of Trinitrotoluene mixed with Ammonium Nitrate is not in any way plastic.
Early attempts to formulate admixtures with Trinitrophenol describe something similar.
A Dictionary of Applied Chemistry Vol 3 - T.E. Thorpe , said patent does not exist as cited.

Attachment: Picric Acid & Ammonium Picrate.pdf (545kB)
This file has been downloaded 840 times
|
|
|
grndpndr
National Hazard
   
Posts: 508
Registered: 9-7-2006
Member Is Offline
Mood: No Mood
|
|
I had problems with the statement "confinement" of picric acid needed to achieve full detonation. All energetics Im aware of benefit from full strong
confinement to achieve full or a max VOD.I dont have any doubts a well milled mix of 10% wax/pet jelly 90% PA crystalls or any other similar
crystaline energetic will detonate with an expected decrease in VOD from the 10% inerts,less than max density given an adequate detonator.Still i
would be suprised if 6500
MPS or therabouts couldnt be achieved with well made dense crystaline PA.
Certainly the mentioned NM/PA det HIGH order w/o confinement and IIRC Nitric acid/Picric acid the same high order det w/o confinement.
Containment or intimate contact all thats required.FWIW I didnt see storage stability mentioned in the OP, what stood out to me was the claim of
"confinement",taken in context it seemed to me PA was useless w/o considerable confinement which we all should know to be untrue..
Ive personally seen well made dense PA crystals High order det In the "confinement" of a large plastic pill bottle.I would call that more akin to a
light containment or intimate contact than a situation of true confinement as its normally used in the context of energetics on this board
FWIW,Take no offense at a differing opinion.
The last portion of your posted patent proves the point in the sense
gums,oils other inert materials were used in PA mixtures even with insensitive AN and did detonate even if the oxidizer portions of the mixture were
dangerous, inerts could be used alone to make a crude
moldable energetic.Certainly not with the plasticizer charateristics of C4 or even semtex but soft enough to mold to the shape of perhaps a RR track
or I beam..If there was some flexible containment it would provide intimate contact with the target object.
For instance ever use a piece of bicycle inner tube filled with an energetic as a line charge on an irregularly shaped object?I assure you it works
well...contained by tamping.
[Edited on 2-8-2010 by grndpndr]
[Edited on 2-8-2010 by grndpndr]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Selected quotations from that same cited reference
A Dictionary of Applied Chemistry Vol 3 - T.E. Thorpe ,

I have no personal experience with picric acid , it has been disused
since the second world war and except as a reagent or dye has
never been available commercially as an explosive to my knowledge.
The question at hand is how reliably will an improvised composition
shoot given the known properties , it seems to require a secondary
as a booster at the least.
A point to note is that the difficulties to detonation appear in the
cast material which paradoxically is at maximum density. Particulate
material of lower density due to the voids present serve to sensitize
it to iniatiation.
[Edited on 2-8-2010 by franklyn]
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
There are so many variables in the methods of measurement of VoD that one can simply give up after awhile of recording the printed figures.
Once I had a lengthy discussion as to the level of strength (greater) of Picric Acid than TNT. I dug up quite a few decent sources for VoD on both and
they were each quite different, even though I was careful as to how the recordings were being measured. I was aware that certain very subtle
circumstances could alter the speed but I simply couldn't find two that matched. The conclusion I reached were that due to differing methods of
production and initiation I was not likely to find conclusive figures. But there were even more complications.
We would all feel comfortable with issues such as density that could alter VoD but some of the most obscure as the placement & position of the
detonation device appears to also have an effect. Often these facts are either not taken into account or not recorded. Placement issues such as one
single compressed block of material vs several blocks with a minute bit of space between the "bricks" will alter speed & on & on it goes.
These complications (density levels) can also affect "confinement". Ideally, when using an energetic material to do work, the placement of the
detonator would be directly in contact with that material & having the greatest surface contact as well [just as the explosive would have
sufficient contact to the object to be moved or cut]. However, the slightest alteration may have a marked difference in the outcome.
My point here is that so many variables exist in examples of "work achieved" from the same explosive; that it's up to the user to do their best to see
what the manufacturer used for his published results.
Deta-Sheet is occasionally used in cracks in concrete to widen a space for a leverage tool or machine to start opening up a huge block so that it can
be broken apart or moved. The crack itself and / any tamping can be viewed as confinement in that instance. However, so much marketing hype has been
part of that product that occasionally even experienced people do not consider it necessary for the pastique to be inserted deeply into the crack (or
that some cracks are just inappropriate). Therefore the POTENTIAL of PETN to shoot at "X" mps becomes almost meaningless due to all the variables
involved.
Sandia Corp. research had found that plastique products with both tackifiers and plasticizers would shoot at 30% inerts (Semtex A, European C6,
Deta-Sheet. etc). What became confusing was that depending upon certain variables, they could shoot as fast as the base material even when adulterated
to that extent. WHY? Sandia concluded it was the density of adjacent energetics resulting from manufacturing (rolled mixing) that placed the
(explosive) particulate in contact regardless of "stand-by" tackifiers / plasticizers as well as the unique quality of those materials to attach the
energetic in direct contact to the work resulting in even greater density/confinement. What occurred to me after reading this was that in such a
situation, density can become confinement.
However, we see the paradox above wherein a cast Picric Acid becomes increasingly dense & confinement in used to detonate it. Does this focus the
initiator (primary)?
I imagine that common physics would say "yes"; it's a issue of focused proximity. But why would than not occur with an example of another cast
nitrobenzene like TNT linear charges?
There are many questions this subject brings up. It would be easy to just chalk it up to peculiarities of Picric Acid; but it may be much more than a
chemical aberration.
1974 Sandia Corp: Characteristics of Non-Military Explosives (Vol. II) R. Liepens. Prepared for Army Mobility Research & Dev Center (166-169)
[Edited on 2-8-2010 by quicksilver]
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Good catch, it could not possible be a British Patent of that
time w/ a 6-digit number, however....
This from Cundill's Dictionary of Explosives. I would have
never found it in my hard copy the index is not that good.
Found it @ Google.com/books
829. III (sub) 2- Roth has patented the use of picric acid (or nitro
bodies containing at least 60 per cent. of picric acid) in
combination with nitrate of ammonia and fatty drying oils. The
explosives so made are to be protected by paper made
waterproof by impregnating it with a mixture of essence of
terebenthine and solid hydrocarbons. (Fr. Spec. No.
173,550, 15.1.86.)
Byda I did check the da 1916 (2nd) and 1922 (3rd)
editions of Thorpe (I own hard copies of the 2 - 4th editions) the
text you posted does not appear in the earlier editions.
Say - anyone know if the last volume(s) of the 4th ed.
were ever published?
[Edited on 2-8-2010 by The WiZard is In]
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
This from :—
Jacques, Daniel
Poudres et explosifs: dictionnaire des matières explosives
1902
Sorry I cannot help you with the French My French couldn't
get me CBT in Madam Dressage's House of Pleasure and Pain.
Roth a fait breveter l'emploi de l'acide picrique (ou des produits
renfermant 60 0/0 au moins de ce composé), en mélange avec
l'azotate d'ammoniaque et des huiles grasses siccatives. L'explosif
obtenu est placé dans des cartouches constituées (le tissu ou de
papier imperméabilisé au moyen d'un mélange d'essence de
térébenthine et d'hydrocarbures solides.
(Brevet français no 173.550, 15 janvier 1886.)
[OCR by Google]
[Edited on 3-8-2010 by The WiZard is In]
|
|
|
grndpndr
National Hazard
   
Posts: 508
Registered: 9-7-2006
Member Is Offline
Mood: No Mood
|
|
Just a quick note the cast material would obviously seem to me to be the most dense.What i have come across for cast Picric acid is 1.64.Other sources
claim it can be pressed to over 1.7 to achieve the often advertised 7480mps.Lends credence to quicksilvers observations different sources different
contributors/circumstances
give a variety of results.FWIW a composite cap Merc ful /PA
the PA in the det was of course pressed to a much higher density that in the pill bottle lightly pressed to an unknown density.As QS said Tamping is
confinement of course in the case of the pill bottle it detonated in a few in of water so some confinement besides the plastic would have been
present.The habit of tamping with sandbags etc.
is also confinement magnifying results which might have skewed
observations/perceptions.
If anyone does try the 10% wax/pet jelly 90% crystaline PA Id sure like to hear about the results,circumstances of use etc.
[Edited on 3-8-2010 by grndpndr]
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by The WiZard is In  |
Good catch, it could not possible be a British Patent of that
time w/ a 6-digit number, however....
Byda I did check the da 1916 (2nd) and 1922 (3rd)
editions of Thorpe (I own hard copies of the 2 - 4th editions) the
text you posted does not appear in the earlier editions.
|
Actually it does ... The text and error in the 2nd - 1916 edition were
carried forward verbatim into the 3rd - 1922 and 4th - 1940 edition.
It only took 94 years for someone to spot the error.
|
|
|
FLchem10
Harmless

Posts: 20
Registered: 29-7-2010
Member Is Offline
Mood: No Mood
|
|
Seems like making a plastic form of PA is possible but if you get a mix wrong by trying some unknown plasticizers you could get a product that can go
very bad!
I just wanted to see if it would make the PA easier to use....I was trying to see if I could make a squib for fun and the models I found seem to be
good designs but I still waiting for my PA to dry!!!
|
|
|
FLchem10
Harmless

Posts: 20
Registered: 29-7-2010
Member Is Offline
Mood: No Mood
|
|
Also just a quick question...Has anyone ever tried to use the PA method using naphthalene instead of asprin....
|
|
|
grndpndr
National Hazard
   
Posts: 508
Registered: 9-7-2006
Member Is Offline
Mood: No Mood
|
|
The synthesis for trinitronapthalene is carried out in steps from mononitronaptalene (also used as a eutectic w/TNP( ,dinitronaphthalene on to
trinitronapthalene.A search of the forum will reveal a synthesis for mono to trinitronapthalene Im sure.A bit more complex as its not a one pot
synthesis but requires 3 seperate nitrations IIRC.On the other hand the intermediate products find uses in cheddites ,one I believe a nitrated
napthalene/chlorate,chlorates the constant some quite powerful on the order of TNT.
[Edited on 4-8-2010 by grndpndr]
|
|
|
FLchem10
Harmless

Posts: 20
Registered: 29-7-2010
Member Is Offline
Mood: No Mood
|
|
Is there a faster way to dry this stuff...and what is a good desiccator compound like "Air dry"
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
An aquarium with a base of calcium chloride or sulfuric acid in open container on the bottom or even an automobile in the hot sun will climb to 60C+.
Shield from UV! Make damn sure the material is washed and as free from acids as possible: check on it with frequency. Provided safety elements are in
place the dessication can be pretty good.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I got a big pail of Silica Gel beads, 5.4Kg with some indicator beads and everything. This was more than I needed, but it is the size they carried
here. It was around $20 for the 5.4Kg, and they are pure silica gel beads. It can be found in the pet section, and is used as a kind of fancy kitty
litter. Apparently with a healthy cat of average weight, the litter only need be changed once a month (it does need to be "mantained" though I guess).
I think this is a good source of silica gel beads, anyone else use this stuff?
I have a couple vaccum containers I got on sale to. They are made to store food in, under vaccum. The containers were meant to go with a big expensive
kit(pump, etc), but I just use my lab pump to evacuate the containers. My question is if I put some of my dry silica gell beads from the oven, in with
the material to be dessicated in the container under vaccum, will it help in the drying process? I am not constantly draughing on the container with
the pump, just pulling a good vaccum then turning a valve on the container and the container holds the vaccum.
Picric Acid is pretty tough stuff. I usually dry it in the oven at around 80C. Unless it is still contaminated with Acid or a lot of sulfate or
sulfo-phenol it should dry quite easily. TNP is not Hygroscopic, so if yours is, it has a contaminant. I keep a close eye on the oven though.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
There are better drying agents in theory, however, theory
doesn't stand a chance up against - I got's...
Lang's Handbook of Chemistry 13th ed.
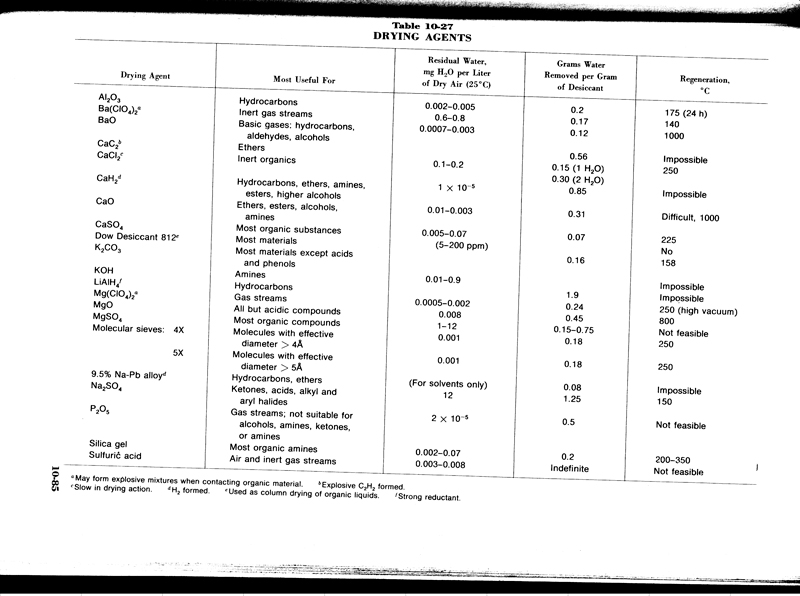
djh
----
There is no higher or lower knowledge, but one only,
flowing out of experimentation.
Leo D.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I don't have alot of experience with desiccants, most things I deal with are not that volatile or sensitive to decomp so I just use the oven. I did do
a little research awhile ago and Silica Gel is widely available (kitty litter), relatively cheap, easily regenerated at relatively low temperature
(reusable), compatible with most chemicals, and very non hazardous in general to use by itself or in combination with other chemicals.
Btw the regeneration temperature in your chart for Silica Gel is on the high side of normal. It can be regenerated at 120C no problem, it will take
longer of course. At the temperatures in the chart the chemical indicators will be destroyed, and the hygroscopic/drying properties can be damaged at
the upper range that the chart states as well.
http://www.apsnyc.com/pdf/silica_gel_reconditioning.pdf
One of many links on dealing with Silica Gel
TNP dries so darn easy, and is not volatile at all, or all that sensitive to decomp(don't go above 120C). Just use a little heat and/or a little
moving air. Remember it melts at around 122C though IIRC.
If you have spent alot of time making nice big dense crystals using heat to dry can degrade the crystals to a certain extent, I think.
What about the silica gel under a vaccum scenario. Is there any advantage to draw a vaccum on your desiccator container, that has your dessicant and
material to be dried in it?
|
|
|
FLchem10
Harmless

Posts: 20
Registered: 29-7-2010
Member Is Offline
Mood: No Mood
|
|
Well I have a gas oven and it just doesnt give a good feel to dry it using a fire heated oven.....
just makes me think of what my kitchen would look like after an exposition haha
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Quote: Originally posted by Hennig Brand  |
I have a couple vaccum containers I got on sale to. They are made to store food in, under vaccum. The containers were meant to go with a big expensive
kit(pump, etc), but I just use my lab pump to evacuate the containers. My question is if I put some of my dry silica gell beads from the oven, in with
the material to be dessicated in the container under vaccum, will it help in the drying process? I am not constantly draughing on the container with
the pump, just pulling a good vaccum then turning a valve on the container and the container holds the vaccum.
. |
It's funny you should bring this up. But to that question let me simply say that I once got a $19 Home-Depot vacuum that I was going to chuck out. I
made a little vacuum, with the idea that the fumes (I was distilling HNO3) would kill the thing after a single distillation & that's all I cared
about anyway. It lasted for months!!!!
You can do all sorts of little things to minimize the wear and tear of course......but I mean to say that I abused it and it held up for a longer time
than i thought. The issue is to try to get access to the diaphragm and see if you can put some material on it to shield it from caustics or pull your
fumes through a bubblier set-up or what-have-you.
If you're just drying stuff out that you've washed and neutralized; I don't see why it shouldn't last for quite along time (PLUS work fine).
On the other hand, if you have a nice one but it's not a PTFE or protected vacuum / pump and you want to make sure that you don't thrash it, then you
are going to have to get into it's guts. There ARE both lubricants and related gell-like substances that are very caustic resistant that will still
allow the pump to function without undue stress.
The real bastard are fumes. If you make your suction pull through liquid so that the fumes don't run through your machine, it will last a long time.
Fumes are the things that mess up the whole enchilada, Fluids can be contained and dealt with, but fumes can find their way into the motor.
[Edited on 7-8-2010 by quicksilver]
|
|
|
grndpndr
National Hazard
   
Posts: 508
Registered: 9-7-2006
Member Is Offline
Mood: No Mood
|
|
I ran across a qoute suposedly from a french patent circa 1885 #167,512. An explosive made by mixing 88 parts of picric acid with 12 parts of melted
paraffin or stearic acid,and then rolling and graining,gives a compact charge when loaded by compression. It is nearly as powerful and brisant as
picric acid,and responds satisfactoriliy to the impulse of the detonator but is distincly less sensitive to mechanical shock.
I expect the patent is referring to compressing into an artillery shell,
but I wouldnt discount complete detonation pressed into a cardboard tube.Contained in an imp EFP or CSC should also provide adequate confinement and
simply tamping the charge will provide confinement.FWIW theres a design floating around for pressing crystaline energetics mixed with binders like
parrafin etc into paper cardboard tube similar to dynamite.
|
|
|
| Pages:
1
2 |