franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Alkane nitration without acid
Interesting but given the reagent one wonders at its utility in general practice
Electrophilic nitration of alkanes with nitronium hexafluorophosphate
http://www.pnas.org/cgi/content/full/94/22/11783
http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=23...
.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
NO3 radical nitration of xylene
Nitrogen trioxide ( NO3 ) charge neutral free radical nitration of an alkene is an
established procedure which by applying the method I outlined here _
http://www.sciencemadness.org/talk/viewthread.php?tid=6717&a...
should be able to produce equivalent nitric esters from benzene ring derivatives
such as xylene. See _ http://en.wikipedia.org/wiki/Xylene
In this case acetone is the prefered solvent being miscible with xylene and
ammonium nitrate. For safety the amount of acetone should be much greater
than what is necessary to solvate a given amount of ammonium nitrate since
in effect one will be subjecting to electrolysis a potentially explosive blend.
The exposure of xylene (CH3)2:C6H4 to NO3 radicals will attach a nitrate group
to each carbon of a cleaved double bond one for each of the six carbons in the
ring , producing dimethylcyclohexylhexanitrate (CH3)2:C6H4(NO3)6 which will
yield upon explosion the products 8CO2 + 2H2O + 3N2 + 3H2
Compared to HMX this is approximately the same size , yet 61.5 % heavier at
478 grams per mole against 296 grams per mole for HMX indicating a greater
density. The carbon of HMX yields 59.5 % dioxide. For this nitric ester ( or is it
a nitric cylcloalkane ? ) derived from xylene it is 73.6 %. This compares favorably
with hexanitrobenzene and octanitrocubane's 75.8 %. Most notably is that HMX
generates 12 moles of gas per mole , same as for octanitrocubane. But this nitric
ester ( or nitrated cycloalkane ) will produce 16 moles of gas in much the same
space as HMX , a 33% improvement.
Given the known properties of nitric esters at best one can only expect this
to be comparable to PETN in sensitivity, or even more so.
A related paper _
Mechanisms and products of the reactions of NO3 with cycloalkenes
http://www.springerlink.com/content/x006510m113h66u5
 Ortho-form Ortho-form
C L I C K F O R F U L L S I Z E ( Then click that image again )
http://img157.imagevenue.com/img.php?image=46692_Dimethylhex...
 Meta-form Meta-form
C L I C K F O R F U L L S I Z E ( Then click that image again )
http://img174.imagevenue.com/img.php?image=46770_Dimethylhex...
 Para-form Para-form
C L I C K F O R F U L L S I Z E ( Then click that image again )
http://img5.imagevenue.com/img.php?image=90776_Dimethylhexyl...
The attached *.zip file contains the Argus lab files of the Ortho-, Meta-, and
Para-form molecules depicted if you care to investigate this further.
[Edited on 3-4-2007 by franklyn]
Attachment: Dimethylhexylhexanitrate.zip (18kB)
This file has been downloaded 580 times
|
|
|
gregxy
Hazard to Others
  
Posts: 421
Registered: 26-5-2006
Member Is Offline
Mood: No Mood
|
|
I don't think an aromatic compund like xylene would add NO3, aromatics tend to substitue instead of adding.
Maybe a highly unsaturated compound like docosahexaenoic acid would be a better choice:
http://en.wikipedia.org/wiki/Docosahexaenoic_acid
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
I just realized that you could make a lot of malonic acid by cleaving that sucker (i.e. DHA) with a lot of ozone.
I digress...
Tim
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by gregxy
I don't think an aromatic compund like xylene would add NO3, aromatics tend to substitue instead of adding. |
Benzene forms a triozonide easy enough, structurally similar
to the Peroxy trimer of Acetone , why should this be different.
Reactive radicals as this only need an Alkene double bond to
cleave. It's an easy enough experiment to do without exotic
reagents, I would try it myself if I wasn't an urban apartment
dweller, which apart from the illegality of it , would be
irresponsible.
.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
1,2,4,5-Tetranitratobenzene
1,2,4,5-Tetrahydroxybenzene (1,2,4,5-Benzenetetrol CAS 636-32-8) ,
http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=6946... / / http://www.chemspider.com/Chemical-Structure.62671.html
forms leaflets from glacial acetic acid (mp 215–220 ºC). It is easily soluble in water, ethanol, and diethyl ether but is
not quite as soluble in concentrated hydrochloric acid and glacial acetic acid. It is obtained by the reduction of
2,5-dihydroxyl-1,4-benzoquinone - http://sioc-journal.cn/EN/abstract/abstract332005.shtml
http://en.wikipedia.org/wiki/2,5-Dihydroxy-1,4-benzoquinone, which is readily made by oxidation of Hydroquinone
(also known as Quinol or Benzene-1,4-diol) - http://en.wikipedia.org/wiki/Hydroquinone , dissolved in strong aqueous
sodium hydroxide with hydrogen peroxide, with stannous chloride and hydrochloric acid or by catalytic hydrogenation ,
US patent 3780114. Etherification with methyl iodide in the presence of base gives the tetramethyl ether of
1,2,4,5-benzenetetrol (mp 103 ºC). Several partial ethers, halo, and amino derivatives of 1,2,4,5-benzenetetrol
are obtained by reduction of the appropriately substituted 1,4-benzoquinones.
Tetrahydroxybenzene Tetraesters US patent 7300604
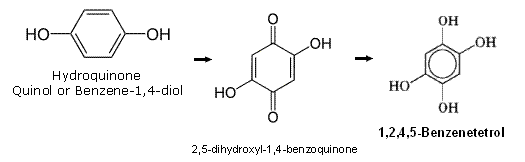
The closing observation above indicates 1,2,4,5-Tetrahydroxybenzene is readily able to be esterfied conventionally
with Nitric and Sulfuric acid to form 1,2,4,5-Tetranitratobenzene. It will be unique , I am not aware of another
aromatic or aryl nitrate. http://www.springerlink.com/content/x006510m113h66u5 . The oxygen balance is just
slightly negative compared to the nearest similar compound Tetranitrobenzene , and will have greater peformance
with it's higher heat of explosion. Tetranitrobenzene being an aromatic nitro compound does have greater insensitivity
to shock.
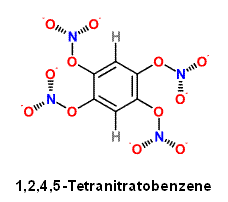
|
|
|
Hoveland
Harmless

Posts: 20
Registered: 20-7-2010
Member Is Offline
Mood: No Mood
|
|
It would appear that melamine does not form stable nitramines.
http://pubs.acs.org/doi/abs/10.1021/ja01195a005
The reference for "dissolved in strong aqueous
sodium hydroxide with hydrogen peroxide, with stannous chloride and hydrochloric acid" did not appear in any of the links you provided. Since these
would be the easiest two routes by far to make the desired compound, it would be very valuable if you could provide a reference for this.
A search failed to reveal anything on benzene nitrate, neither on carbolic-nitric anhydride, which strongly suggests that benzene nitrate is not
stable and does not exist in macroscopic quantities (at reast at RT). There is strong reason to believe that a nitrate cannot be put on a benzene
ring, but that is discussed elsewhere on the forum.
2,5-dihydroxy benzoquinone does seem like a very useful precursor to make other compounds. (condensation with hydroxylamine?)
[Edited on 23-7-2010 by Hoveland]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
@ Hoveland
" The reference " is here , Page 27 , 4.3
Attachment: Polyhydroxy Benzenes.pdf (475kB)
This file has been downloaded 1032 times
Carboxylic acid seems to attach nicely , as an acid chloride , page 9
click to obtain =>
http://www.google.com/patents/download/7300604_Tetrahydroxyb...
1,2,4,5-benzene-tetrasulfonic acid is the exact analog of what I outlined
http://www3.interscience.wiley.com/journal/123471062/abstrac...
( 1,2,4,5-tetrasulfonic acid is prepared in two steps starting from 1,2,4,5-Tetrachlorobenzene )
and it is even suggested as a stabilizer for hydrogen peroxide of all things. ( see line 18 )
http://www.google.com/patents/download/4744968_Stabilized_aq...
Clearly one can just start from that instead to get the o-nitrated tetraester.
Aryl chemistry is bewildering in its variations and dependant on the specific
arrangement of attached groups. There is a zoo of sulfonic benzenes each
with distinctive properties which cannot be generalized.
http://www.chemsynthesis.com/base/chemical-structure-14329.h...
http://www.gfschemicals.com/statics/productdetails/DIHYDROXY...
http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=8508...
http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=2630...
http://us.chemicalbook.com/ChemicalProductProperty_US_CB5542...
When they may be hydrolytically liable they are isolated as salts. I think that
your concerns are based on the more common lesser sulfonated variants. As
observed below.
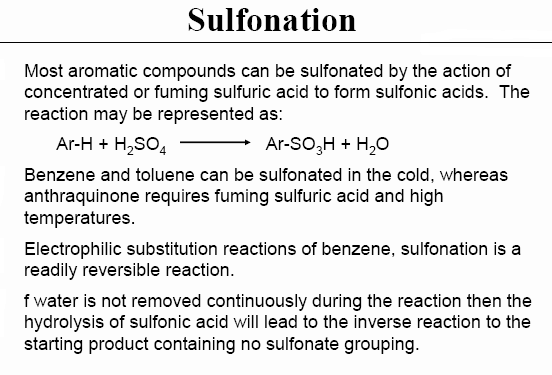
[Edited on 23-7-2010 by franklyn]
|
|
|