Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Vitamin To Valuable Precursor
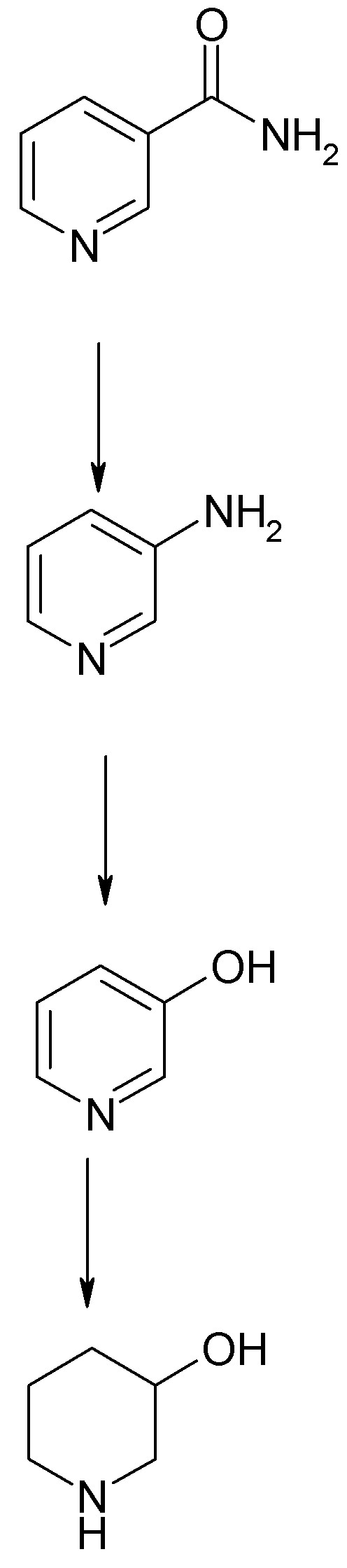
The ubiquitous vitamin Nicotinamide (niacin) is nicotinic acid amide or pyridine-3-carboxamide. I have a Kg of pharm grade purchased from a Roche
distributor a few years ago.
This can be converted easily to 3-aminopyridine by Hoffmann degradation using hypochlorite or hypobromite in strong basic solution. See the attached
Org.Syn. procedure.
3-aminopyridine is readily diazotized to 3-hydroxypyridine and the hydrochloride of this compound is readily hydrogenated to 3-hydroxypiperidine. In
the hands of Biel and coworkers 39.5 g of 3-hydroxypyridine hydrochloride was reduced to a mixture of 9 g 3-hydroxypiperidine and 13,5 g piperidine
(via hydrogenolysis of the -OH group> Conditions were mild: 60 psi H2, 2.2 g
PtO2 catalyst in a Parr shaker at room temperature. See JACS 74 p.1487 (1952). I believe this modest yield can be improved through judicious
experimentation with catalyst. Conditions were mild: 60 psi H2, 2.2 g
PtO2 catalyst in a Parr shaker at room temperature. See JACS 74 p.1487 (1952). I believe this modest yield can be improved through judicious
experimentation with catalyst.
The significance of this compound become clear if you read the threads on Ditran and related esters of N-alkyl derivatives of this alcohol.
Quaternized salts lose all psychotomimetic activity but are useful spasmolytics.
Of course 3-hydroxypyridine can be purchased but it is a little expensive so this two step procedure from the vitamin is potentially very convenient,
isn't it?
There are other pathways to these hydroxypiperidines which we can examine but none of them as OTC as starting from nicotinamide.
[Edited on 19-7-2008 by Sauron]
Attachment: CV4P0045.pdf (140kB)
This file has been downloaded 726 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
1. Hoffmann Degradation w/ Hypohalite Yield 65-71%)
2. Diazotization and Hydrolysis
3. Reduction as Hydrochloride yield 25% obviously weak link
Ready for N-Alkylation (Yield 82% for N-ethyl) and Esterification
[Edited on 19-7-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
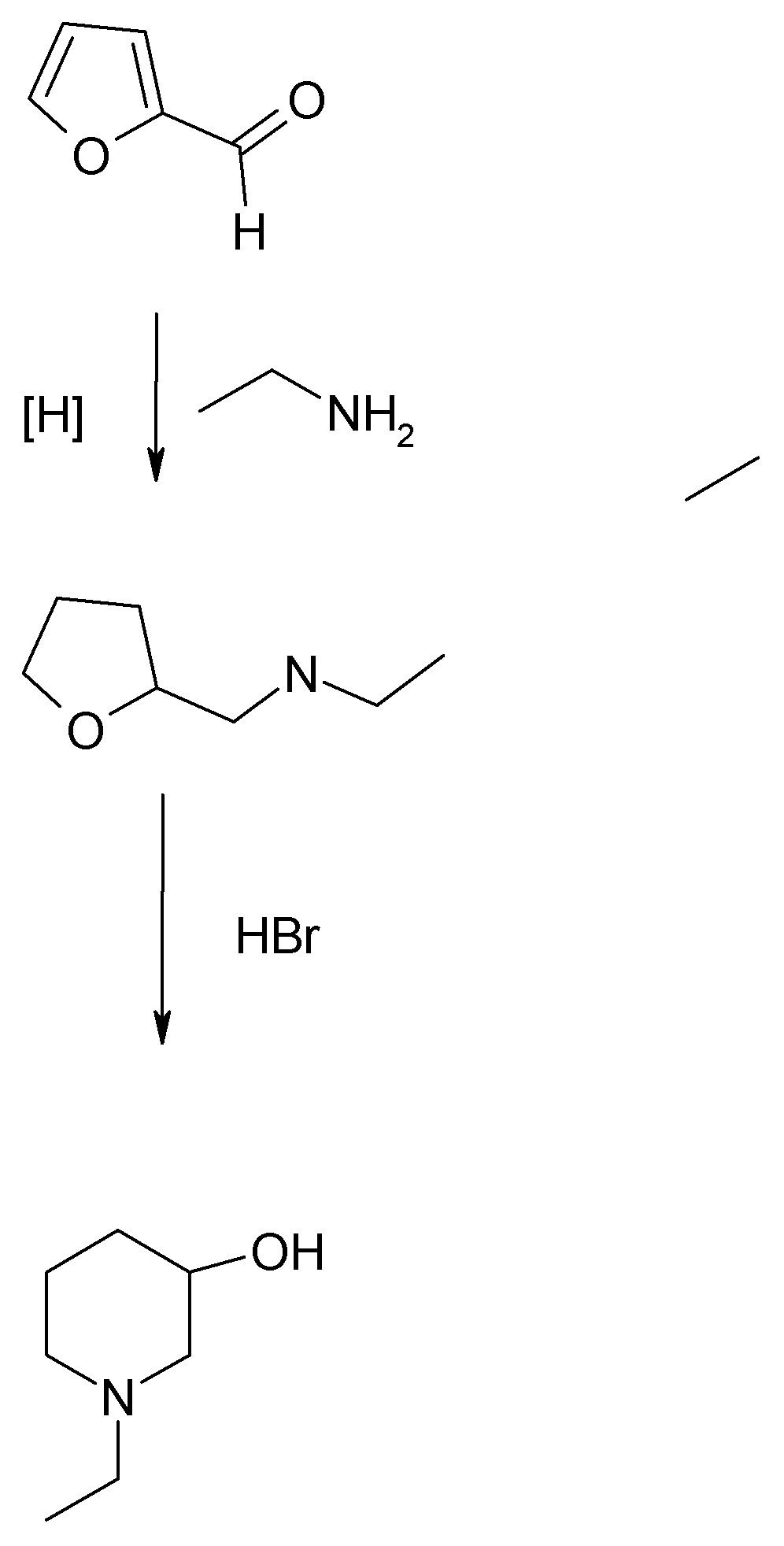
The method used by Biel and coworkers at Lakeside Labs in the late 40s and 50s/60s was based on the discovery by Paul and Tchiletcheff (see French
paper posted in More on CW threads). This involved reductive amination of furfural or preferably, tetrahydrofurfurl chloride, with a monoalkylamine or
dialkylamine. The furfural feedstock gave lower yields. Tetrahydrofurfuryl chloride can be prepared from furfural by catalytic reduction followed by
chlorination of the tetrahydrofurfuryl alcohol. Both the THF-methanol and its chloride are commercially available, while furfural is both common
commercially and may be readily prepared by dilute acid hydrolysis of a wide variety of agricultural wastes. (See Org.Syn. for procedure from dried
corn cobs.)
The product is N-alkyl-3-hydroxypiperidine. In the instance of N-ethyl-3-hydroxypiperidine starting from furfural and ethylamine, using Bie's
improvement of the French process, an overall yield for the two step procedure was better than 50%. Using the original instructions of Paul, only
about 26%.
Typical conditions are 100 atmospheres H2 and moderate temperatures over Raney nickel so a suitable autoclave is a must.
It is clear that the "French furan" method is the way to go if one has the requisite high pressure hydrogenation apparatus.
If not then the lower yielding route from nicotinamide which only calls for 60 psi, and can be performed in a glass pressure bottle of the Parr type
in either a shaker or with magnetic stirring, is doable, albeit more steps.
[Edited on 19-7-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
roamingnome
Hazard to Others
  
Posts: 363
Registered: 9-9-2006
Member Is Offline
Mood: No Mood
|
|
Well this is out of character.
but when i googled, i found the russian hive.
http://narkopedia.hit.bg/natural/delirianti/delirianti.htm
it seems like a natural molecule to some extant? Or has natural precursors.... An interesting side project for a smoothly running lab.
But i think this candidate lost during the elections of 66' to lsd. I think you can still find old buttons in thrift stores that say
" Vote DITRAN 1966'"
[Edited on 20-7-2008 by roamingnome]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
If you stay away from N-Me and N-Et you avoid legal hassles. If you stay away from benzilic acid likewise. And if you quaternize the N, the substance
loses all of its delerium inducing properties and is just a spasmolytic.
There are pharm companies doing exactly this.
So I think you jump to the wrong conclusions.
Anyway, anyone who thinks Ditran is a fun recreational is a sick puppy.
Sic gorgeamus a los subjectatus nunc.
|
|
|
un0me2
aliced25 sock puppet
  
Posts: 205
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
Sauron, I hate to mention it, but you are the one who came up with it and originally the N-ethyl-3-piperidinol at that... That and Benzillic acid (in
a spray bottle full of DMSO) in the wrong/right hands would surely make the job of riot police harder... That'd teach the fuckers to fire tear gas/CN
wouldn't it?
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Thank you for your post Sauron. There have been fewer of them lately, and I have missed your input.
I appreciate your enthusiasm for chemistry, and your willingness to research subjects, and then share your findings with others. Kudos.
[Edited on 22-7-2010 by zed]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Look at the date that post was made... 2 years ago!
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
So?
|
|
|