un0me2
aliced25 sock puppet
  
Posts: 205
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
The Phenacylamines - would Quinacetephenone work?
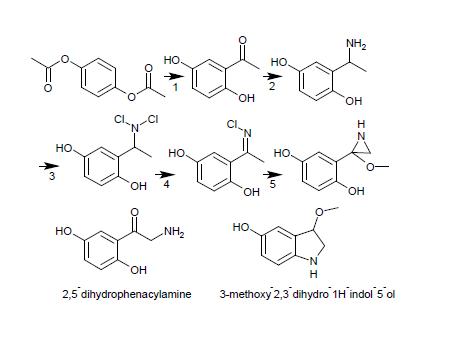
Now Orgysn has a procedure whereby the Friedel-Crafts acylation/Fries Rearrangement/etc. is used to make quinacetophenone (aka 2,5-dihydroxyacetophenone) from Hydroquinone, diacetate (there is an alternative preparation using the Nencki Reaction, which uses Glacial Acetic Acid & Fused Zinc Chloride). Orgsyn also has a procedure whereby Phenacylamine Hydrochloride is prepared from the rearrangement of the 1-Phenethylamine/a-phenethylamine to the a-aminoacetophenone.*
Now, the question is, will the procedure work with 2,5-dihydroxyacetophenone/2,5-dimethoxyacetophenone? The reason I ask is there is a real risk of
cyclization (to my mind), with either the 2 or the 5 hydroxyl(s). However, if it does, reduction of the product (as per Rappoport, et al &
numerous others, ~4 Atm H2/10% Pd/C) should give the 2-CH in a fairly direct route...
The other alternative is via Sartori's procedure for the chlorination/bromination of the acetophenone (yeah, I'd rather avoid that - homemade CN at reflux yummy, I'm sure I'd be popular with the missus and the rest of the suburb ), then using that to alkylate ammonia/alkylammonia. ), then using that to alkylate ammonia/alkylammonia.
* There is another paper of interest (attached) where the dichlorination of the amine is done in a much easier manner using potassium hypochlorite.
Attachment: Baumgarten.Bower.Reactions.of.Amines.I.A.Novel.Rearrangement.of.NN.Dichloro.sec.Alkylamines (558kB)
This file has been downloaded 1426 times
[Edited on 11-7-2010 by un0me2]
|
|
|
Methyl.Magic
Hazard to Others
  
Posts: 139
Registered: 14-5-2007
Member Is Offline
Mood: No Mood
|
|
for dimehoxyphenacylamine you can methylate the dihydroxyacetophenone with TMP or whatever then brominate the alpha position of the ketone with CuBr2
and swap the bromo with NH2 with aqueous ammonia (good yield expected IMO).
|
|
|
un0me2
aliced25 sock puppet
  
Posts: 205
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
I'd prefer the rearrangement, if it is possible, I'd rather not play with a-chloro- or a-bromo-acetophenones (CN just has that ring to it). In terms
of CuBr2, CuCl2 should also do the same thing, but why bother when straight bromine/chlorine will do the job? Here is the preparation of the
a-chloroacetophenone/phenacyl chloride (from Sartori, "The War Gasses", p.156:
LABORATORY PREPARATION
It is prepared by the action of chlorine on acetophenone according to Korten and Scholl's method. 20 gm. acetophenone and 100 gm. acetic acid are
placed in a flask fitted with a stopper carrying two holes, through one of which passes a delivery tube for the chlorine and through the other an
air-condenser. The mixture is agitated to facilitate the solution of the acetophenone and then the whole is weighed. A rapid stream of chlorine is
passed through the solution, cooling externally if necessary until the necessary amount of chlorine has been absorbed.
The product is allowed to stand at ordinary temperature until the liquid becomes colourless. It is then poured into ice-water; the chloroacetophenone
separates as an oily liquid which rapidly solidifies. The crystals are separated and crystallised from dilute alcohol.
Now that is better than fucking around with multiple solvents, Cu(II) salt preparation, etc. It is still shite because it entails dealing with
substituted CN gas. As to the yield, I'm unsure about the yield quite frankly...
|
|
|
Vogelzang
Banned
Posts: 662
Registered: 26-4-2008
Member Is Offline
Mood: No Mood
|
|
There's a lot of information about the Fries rearrangement here
https://www.hyperlab.info/inv/index.php?s=&act=ST&f=...
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
You mean, if it would work with 1-(2,5-dihydroxyphenyl)ethylamine? No, it would not. This is a phenol and furthermore a hydroquinone, so you can't put
it trough such a treatment (none of the steps is applicable). I even doubt it would work with 1-(2,5-dimethoxyphenyl)ethylamine, though with this
substrate only the first step is problematic.
| Quote: | The other alternative is via Sartori's procedure for the chlorination/bromination of the acetophenone (yeah, I'd rather avoid that - homemade CN at reflux yummy, I'm sure I'd be popular with the missus and the rest of the suburb ), then using that to alkylate ammonia/alkylammonia. ), then using that to alkylate ammonia/alkylammonia. |
I don't know what is your problem with phenacyl chlorides/bromides. I made ~25g of phenacyl chloride in an apartment when I was younger and more
irresponsible. No big deal. I will not deny that there were tears involved in the process, but so what? You can use a gas mask if it annoys you.
Besides, you can not alpha-halogenate 2,5-dihydroxyacetophenone. It is a phenol, like I already said. You can however alpha-halogenate
2,5-dimethoxyacetophenone. The resulting substituted phenacyl halides don't have much of a vapour pressure at room temperature, so you don't have to
cry just because you succeed in synthesizing them. Here are a few examples on 2,5-dimethoxyacetophenone:
Synthesis (1988) 545-546.
Bioorganic & Medicinal Chemistry Letters, 18 (2008) 1297-1303.
Chemical & Pharmaceutical Bulletin, 40 (1992) 1170-1176.
What is the reference for the synthesis of alpha-aminoketones by alkylating ammonia with alpha-haloketones? This is completely counterintuitive and I
don't see how this could be possible. The reaction of alpha-haloketones with ammonia should give dihydropyrazines. The nearest compounds to primary
alpha-aminoketones that are isolable are the alpha-ammoniumketones. These can be synthesized from alpha-haloketones, but only applying methods that
skip the intermediacy of aminoketones. Such are hydrogenation of alpha-nitrosated ketones or alpha-azidoketones in the presence of HCl and the
Delepine reaction with the hydrolysis step performed with HCl(aq). For examples on 2,5-dimethoxyphenacyl halides to give 2,5-dimethoxyphenacylammonium
chloride, see:
Chemistry-A European Journal, 13 (2007) 7780-7784. (via azide)
WO2004018409 (via Delepine reaction)
Journal of Medicinal Chemistry, 47 (2004), 6034-6041. (Delepine reaction on a related substrate, 4-bromo-2,5-dimethoxyphenacyl bromide)
But to obtain a 2,5-dimethoxyphenacyl halide it makes much more sense to just acylate 1,4-dimethoxybenzene with chloroacetyl chloride. With this you
have one step instead of many. Definitively read the Hyperlab [post=534107] and [post=534135]. It appears to me you missed those or else you would not
be lost in your alternative routes.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
un0me2
aliced25 sock puppet
  
Posts: 205
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
Nicodem, all is not lost I say , here is the preparation of some Ring Oxygenated Phenacyl Bromines (by bromination of a variety of alkoxy substituted acetophenones - paper
attached (Chem. Pharm. Bull. Vol.26(10) 1978 pp.3218-3222). , here is the preparation of some Ring Oxygenated Phenacyl Bromines (by bromination of a variety of alkoxy substituted acetophenones - paper
attached (Chem. Pharm. Bull. Vol.26(10) 1978 pp.3218-3222).
Then here is a paper (YAKUGAKU ZASSHI, Vol.76(9) 1956 pp.1094-1096/attached) on the alkylation of substituted amines (most probably a-phenethylamine
(OTC), which can be removed ala Nichols, Rappoport, et al) with phenacyl bromide (prepared differently, but all the same).
When you say only the first step would be problematic (presumably the rearrangement of the phenethylamine) with the dimethoxyaminoketone, why is that?
There is a paper on this site where the same result is gained on a p-methoxyphenethylamine with TCCA.
With potassium hypochlorite I was hoping to avoid any demethylation problems (if the methylated substrate had to be used).
On the contrary, I was VERY unsure that the ring-methoxyl groups would survive the rearrangement itself. I am still rather interested in the
concept...
NB I am trying to avoid building methylation in prior to as many low-yielding steps as possible. I know that people here (and elsewhere) have
EXTREMELY little access to methanol (thus making it a limiting reagent and the 'unusual' synthesis route).
PS If I were to design the shortest simplest route possible, I'd use Effenberger's paper/patent, go via the FC Rxn of N-Phthalloyl-glycinyl chloride
on 1,4-dimethoxybenzene, then proceed to nitrate the 4-position (with the protecting group in place), Sandmeyer to the
N-Phthalloyl-4-Cl-2,5-dimethoxyphenethan-1-one-2-amine (Dr Effenberger actually describes the use of this with the 2,5-aminoketones). I'm simply
trying to work out a route using the smallest possible amount of equipment.
Attachment: Fujii.Yoshifuji.Ohba.Preparation.of.Some.Phenacyl.Bromides.pdf (747kB)
This file has been downloaded 620 times
Attachment: Abe.Yamamoto.Sato.Synthesis.of.a.3.5.Dimethoxyphenyl.B.monomethylaminoethanol.A.Uterus.Contracting.Agent.pdf (499kB)
This file has been downloaded 708 times
Attachment: Effenberger.Steegmuller.FC.Acylation.N.PhthalloylAlanine.pdf (706kB)
This file has been downloaded 602 times
Attachment: US4945168.Effenberger.EtAl.Method.of.Preparing.Aryl.1.Phthalamido..Alkylketones.pdf (297kB)
This file has been downloaded 640 times
PPS Hang on, I might be onto something here, Baumgarten, et al, are claiming that rearrangement of 1-phenpropylamine (from propiophenone) rearranges
to the cathinone via rearrangement of the N-Chloroketimine... Interesting...
[Edited on 12-7-2010 by un0me2]
|
|
|
un0me2
aliced25 sock puppet
  
Posts: 205
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
Holy fucking hell, I am reading this right yeah?
R1CH(NHCl2)CH2R2 =base + acid hydrolysis => R1COCH2(NH2)R2
Means that if R1=C6H5 & R2=CH3, then they are in fact dealing with the
rearrangement of 1-phenyl-1-aminopropane to 2-amino-1-phenyl-1-propanone?
I'm looking for problems, but I'm not seeing anything more than the amine moving from the 1-carbon to the 2-carbon, with side-chains of 2-whatever in
length, to form the relevant aminoketone. Also tolerant of "some" substituents on the ring.
Damn, if that is right, then propiophenone + ammonium formate ==> phenyl-1-aminopropane + TCCA ==> N,N-dichloro- + base ==> Azirine + HCl
==> Cathinone.
That is dangerously simple
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
Make the quinacetophenone and a-brominate it with CuBr2.
Next you can take 3 different paths.
You can make the amine via delepine synthesis
You can make the amine via the gabriel synthesis, but I worry hydrolysis of the phenacyl phthalimide could be messy in this case.
You can make, the azide by stirring with an excess of NaN3 in DMSO or MeCN overnight. And then reduce it by A) Staudinger Reaction or B) via
reduction with pd/c under atm pressure in Et2O saturated with HCl. The reduction will proceed vigorously and then stop without carbonyl reduction due
to great insolubility of the hydrochloride (even if the starting material isn't all that soluble in ether).
I would pick the later with the Pd/c reduction because, the azide is generally obtained pure without any purification, and the amine hydrochloride
should be obtained in fair purity by adding enough methanol to get the hydrochloride dissolved and filtering through celite.
p.s. with reduction of azides, since an equivalent of nitrogen is produced for each eq of H2 consumed, using a very large excess of H2 or refilling
the balloon after the reduction seems to have slowed down is sometimes required.
[Edited on 7-12-2010 by smuv]
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by un0me2  | Nicodem, all is not lost I say , here is the preparation of some Ring Oxygenated Phenacyl Bromines (by bromination of a variety of alkoxy substituted acetophenones - paper
attached (Chem. Pharm. Bull. Vol.26(10) 1978 pp.3218-3222). , here is the preparation of some Ring Oxygenated Phenacyl Bromines (by bromination of a variety of alkoxy substituted acetophenones - paper
attached (Chem. Pharm. Bull. Vol.26(10) 1978 pp.3218-3222). |
I told you it is easy. Check the three references for the halogenation of 2,5-dimethoxyacetophenone that I gave you above. Two are about the
brominations and use simple conditions.
I was not aware that CuBr2 was successfully applied on 2,5-dihydroxyacetophenone (the reference posted by smuv above). I only searched for the
synthesis of 2,5-dihydroxyphenacyl chloride found none that involved direct chlorination. However, I do not follow your logic. You can not make any
nucleophilic substitutions on 2,5-dihydroxyphenacyl bromide with basic N-nucleophiles or else you end up with the corresponding benzofuranone. The
only N-nucleophile that might have a low enough basicity is the azide, only perhaps though, because I have seen this cyclisation done with sodium
acetate (pKa similar to azide!).
At which point would you methylate anyway? In general, I find it somewhat obnoxious when people start to complicate synthetic routes. I was trained to
do the opposite, so any such discussion is frustrating for me. But don't get me wrong, I do not consider such discussion useless!
| Quote: | | Then here is a paper (YAKUGAKU ZASSHI, Vol.76(9) 1956 pp.1094-1096/attached) on the alkylation of substituted amines (most probably a-phenethylamine
(OTC), which can be removed ala Nichols, Rappoport, et al) with phenacyl bromide (prepared differently, but all the same). |
Yes, there are thousands of alkylations of primary and secondary amines with alpha-haloketones, but I found only one patent and one paper, both weird
and unreliable examples for the alkylation of ammonia with phenacyl chlorides that yield phenacylamines (and none with phenacyl bromides).
| Quote: | | When you say only the first step would be problematic (presumably the rearrangement of the phenethylamine) with the dimethoxyaminoketone, why is that?
There is a paper on this site where the same result is gained on a p-methoxyphenethylamine with TCCA. |
No, the rearrangement should go smoothly with the synthesis starting from 1-(2,5-dimethoxyphenyl)ethylamine, it is the N-dichlorination step that I'm
not sure. The closest example in that Org. Synth. paper is the synthesis of p-methoxyphenacylammonium chloride. With a substrate as activated as
1-(2,5-dimethoxyphenyl)ethylamine there are good chances that there would be some ring chlorination during the N-chlorination step (though this is
highly depending on the electrophilicity of the chlorinating reagent and the solvent used - there should be conditions where this can be avoided).
| Quote: | | With potassium hypochlorite I was hoping to avoid any demethylation problems (if the methylated substrate had to be used). |
I'm afraid I do not follow. What demethylation problems?
| Quote: | | NB I am trying to avoid building methylation in prior to as many low-yielding steps as possible. I know that people here (and elsewhere) have
EXTREMELY little access to methanol (thus making it a limiting reagent and the 'unusual' synthesis route). |
You are not considering this synthesis in a holistic manner. It is not wise to chose to work on a substrate that gives a lower yield at each step, if
it gives the desired product at all, just because you think a single step is problematic in the original idea. The logic in organic synthesis is in
most cases to chose the route that is the most straightforward and uses the most robust substrates and possibly only reactions with a wide scope.
Sometimes less general reactions with a very narrow scope provide a shorter synthetic route, but in such case things get more challenging and require
much more research, just to skip a step or two.
| Quote: | | PS If I were to design the shortest simplest route possible, I'd use Effenberger's paper/patent, go via the FC Rxn of N-Phthalloyl-glycinyl chloride
on 1,4-dimethoxybenzene, then proceed to nitrate the 4-position (with the protecting group in place), Sandmeyer to the
N-Phthalloyl-4-Cl-2,5-dimethoxyphenethan-1-one-2-amine (Dr Effenberger actually describes the use of this with the 2,5-aminoketones). I'm simply
trying to work out a route using the smallest possible amount of equipment. |
That is not short. Check the above mentioned Hyperlab posts.
| Quote: | Damn, if that is right, then propiophenone + ammonium formate ==> phenyl-1-aminopropane + TCCA ==> N,N-dichloro- + base ==> Azirine + HCl
==> Cathinone.
That is dangerously simple |
Simple? Say that again once you try it out. How could that be simple in comparison to the most classical synthesis of cathinone?
(alpha-nitrosation of propiophenone followed by hydrogenation in the presence of HCl - two very trivial, simple and robust steps)
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
un0me2
aliced25 sock puppet
  
Posts: 205
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
Well, if nothing else, there is some serious discussion going on, that can't be a bad thing in and of itself, no?
Now, I have found some more Japanese papers where they react the 2-phenylazirine with various reagents, including phthalic anhdyride to get the
N-Phthalloyl-aminoketone. I'm thinking that the phenacyl bromide/chloride should react with phthalimide to give the N-Phthalloyl-aminoketone, as used by Shulgin, Nichols, etc (They also used other acids, like benzoic anhydride to get the
N-Benzoyl-aminoketone).
PS I'm not purposefully trying to make things harder mate, I'm trying to make things simpler for people without access to chemical supply companies.
All I'm doing is trying to take 2-CH out of the "too-hard" basket for some people.
PPS Is there any papers you know of involving the decarboxylation of, preferably, iodoacetic acid to iodomethane? I'm looking about now, that would
solve one major issue, it may be in the labeled compounds...
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
| Quote: | | ...benzofuranone... |
This was a foolish mistake on my part, I have actually past experience with this side reaction.
| Quote: | | The only N-nucleophile that might have a low enough basicity is the azide, only perhaps though, because I have seen this cyclisation done with sodium
acetate (pKa similar to azide!). |
I have prepared a 2'-hydroxy-phenacyl azide. Yields are good, the product is base sensitive (probably b/c of cyclization). I prepared the azide by
stirring the phenacyl chloride with an excess of NaN3 for a few hours with 90% MeCN/DMSO.
| Quote: | | All I'm doing is trying to take 2-CH out of the "too-hard" basket for some people. |
Next time you would waste a lot less of other people's time if you simply asked that.
[Edited on 7-12-2010 by smuv]
[Edited on 7-12-2010 by smuv]
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
un0me2
aliced25 sock puppet
  
Posts: 205
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
smuv
I didn't realise I'd been that opaque... The rearrangement of 2,5-dihydroxyacetophenone to the 2,5- aminoketone would surely have (especially given I
have drawn a reaction schema) have got that point across. But I do apologise for wasting anyone's time, I was merely thinking of an alternative
approach to a multi-step synthesis, which doesn't use anywhere near as much in the way of hard-to-get chemicals and/or equipment.
Thank you for your assistance in any event, I am sorry if I have misled you in any way shape or form. I personally hate others wasting my time so I
know how you feel.
Nicodem
Nicodem, the demethylation I was worried about is that that 'can' accompany the oxidation of hydroquinones - both persulfate and Fremy's radical show
that they cause demethylation during the oxidation of phenols to hydroquinones.
I honestly hadn't considered the potential for ring-chlorination, I have read the relevant syntheses and based upon those, imagined ring-chlorinated
products should be rather harder to build. In fact, I'd be more than happy to have ring-chlorination of this substrate (particularly regiospecific
ring-chlorination ), although I suspect you are talking about rather less
controlled halogenation than would be beneficial. ), although I suspect you are talking about rather less
controlled halogenation than would be beneficial.
I'm unsure how the Hyperlab procedures could be shorter than Effenberger's FC Acylation with the N-Phthaloyl- amino acid chlorides... He used
1,4-dialkoxybenzene to get the N-Phthaloyl- 2,5-dialkoxy- amino ketone in a single step. As this intermediate is the one used by many researchers in
order to introduce the 4-Halo group (whether by direct halogenation or via the nitration/reduction/Sandmeyer), I really don't see how a shorter route
is possible.
I am intrigued by your statements regarding the N-Succinimide based scheme(s), how is the protecting group removed? Presumably the synthesis itself is
essentially that of Gabriel, but I'm unable to find anything much on the removal of the N-succinoyl group, do you happen to have any of the papers you cite on this?
Would the N-Succinoyl-amino acids be capable of being used in the FC Acylation of the dialkoxyhydroquinone? Would the N-Succinoyl group survive the
nitration/diazotization of the substrate (like the N-Phthaloyl- group).
I'm also waiting on several papers that I have requested - they may or may not show the use of this (the "N-chloroimine-modified Neber reaction"*) on
similar substrates.
PS I'm considering asking a friend about their farm-use 'bio-diesel' setup, I'll have to check to see if they are licensed** to purchase methanol and
if not, see if they can become so licensed
* I've uploaded two papers where the mechanism of the N-chloroimine rearrangement is examined and determined to be essentially the same as the
"N-Tosylimine Neber Rearrangement", with the added benefit that TCCA/Hypochlorite are a lot easier to source than TsCl.
** Sad as it is, without a license you can expect unwanted attention. Biodiesel is well enough known now that it should not attract anywhere near the
attention of simply trying to purchase methanol without it.
PS I honestly don't think that time spent examining and then either accepting or rejecting various schemes is wasted. I honestly cannot say I've seen
anything much on this, so the input from the various posters is extremely useful (and gratefully received). I am not being deliberately obtuse, I am
simply asking questions that I feel need to be asked, despite knowing how dumb I may look for having asked certain questions.
Attachment: Alt.Knowles.The.Mechanism.of.the.NN.Dichloro.sec.Alkylamine.Rearrangement.pdf (355kB)
This file has been downloaded 719 times
Attachment: Nakai.Furukawa.Oae.The.Mechanism.of.the.Base.Promoted.Rearrangement.of.NN.Dichlorocyclohexylamine.to.2.Aminocyclohexanon (438kB)
This file has been downloaded 1056 times
PPS After posting I finally found a hit on the modified Gabriel Amine Synthesis using succinic acid/the succinimide (from phenacyl chloride) which
describes the removal of the N-succinoyl group in 90+% yield (attached).
[Edited on 12-7-2010 by un0me2]
Attachment: Augustine.Tanielyan.Marin.Alvez.Synthesis.of.Chiral.2.Amino.1.Phenylethanol.pdf (318kB)
This file has been downloaded 2660 times
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
Quote: Originally posted by un0me2  | smuv
The rearrangement of 2,5-dihydroxyacetophenone to the 2,5- aminoketone would surely have (especially given I have drawn a reaction schema) have got
that point across. |
I am not on this forum to read minds, I am here to learn/practice chemistry.
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Quote: Originally posted by un0me2  |
NB I am trying to avoid building methylation in prior to as many low-yielding steps as possible. I know that people here (and elsewhere) have
EXTREMELY little access to methanol (thus making it a limiting reagent and the 'unusual' synthesis route). |
What's the purpose with all this chemical masturbation? You want to make N-protected amino acid chlorides and use Pd/C hydrogenation but don't know
where to get methanol?
|
|
|
un0me2
aliced25 sock puppet
  
Posts: 205
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
Oh, I know where to get it, just don't feel like appearing on "the list" in such a small community is all.
Ncodem is right about the succinimide - see the attached paper, where they form 2-amino-1-phenylethanol from phenacyl chloride and succinimide.
As to chemical masturbation, call it what you will... I still reckon I'll be able to come up with a procedure that avoids nitromethane/nitroethane and
fucking benzaldehydes... I also avoid using N-protected amino acid chlorides, providing the 2,5-diOX-acetophenone can be chlorinated effectively,
seems like a better set of conditions than I've seen cited to date.
The azirine can be opened with acids to give the N-acylamino ketone (thus avoiding the need for strong base - see the 2nd paper). The only trouble is
I don't believe that the N-succinylamino ketone could be nitrated/etc. given that it is so easily removed.
The only hard to get chemical here (apart from methanol for moi) is the acetic anhydride for the initial step (S2Cl2 is looking good, even if it is impure, it should be clean enough).
Attachment: Sato.Kato.Ohta.Azirines.II.The.Reaction.of.2.Phenylazirine.with.Acylating.Agents.pdf (534kB)
This file has been downloaded 805 times
|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
If you dont want to use nitromethane or nitroethane you could get your amine from an amide or nitrile, but that doesnt seem any easyer that going from
and aldehyde and nitroalkane.
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
| Quote: | | The azirine can be opened with acids to give the N-acylamino ketone (thus avoiding the need for strong base - see the 2nd paper). The only trouble is
I don't believe that the N-succinylamino ketone could be nitrated/etc. given that it is so easily removed. |
No need to open the aziridine, H2 Pd/c will catalyze the hydrogenolysis of both the aziridine and the methoxy ether to form the desired
phenylethylamine. From my experience with somewhat analogous 2-phenyl-2-methyl-dioxolanes, these can be reduced under atmospheric pressure with 5%
pd/c in AcOEt. Benzylamines are a little harder to reduce, but might be doable under atmospheric pressure.
Read a book about catalytic hydrogenation for more details.
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
I have recently uploaded some such books, and posted the links for downloading them in the Organic thread in the References section.
[Edited on 13-7-10 by JohnWW]
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Quote: Originally posted by un0me2  |
As to chemical masturbation, call it what you will... I still reckon I'll be able to come up with a procedure that avoids nitromethane/nitroethane and
fucking benzaldehydes... I also avoid using N-protected amino acid chlorides, providing the 2,5-diOX-acetophenone can be chlorinated effectively,
seems like a better set of conditions than I've seen cited to date.
|
Every speed-freak can post all day about all these wonderful insights and ideas without saying anything useful. Well, the Friedel-Crafts route is
nothing new, even bk-2C-B has been made by Glennon, doi: 10.1021/jm040082s. The amine function can be introduced using hexamine, which you can get
from you local shop -- no need for azirine-based rearrangements. However, you still need to make the (a-halo)acetophenone, as well as to get rid of
that keto group and this is not as easy and OTC as going from hydroquinone via p-methoxyphenol, ortho-formyation, methylation, nitromethane, Zn/HCl
reduction. That this is the case should be obvious, as everyone still prefers the nitrostyrene route.
|
|
|
un0me2
aliced25 sock puppet
  
Posts: 205
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
Smuv
Here is the file you referred to (I actually uploaded it to THIS site once before regarding propiophenone). I also added another which uses CuCl2 to
give the 2-chloroketone (though Shulgin uses it in the 2-CC/DOC synthesis from memory, for ring-chlorination IIRC).
Attachment: King.Ostrum.Selective.Bromination.with.Copper(II).Bromide.pdf (391kB)
This file has been downloaded 670 times
Attachment: Kosower.etal.Halogenation.with.Copper(II).I.Saturated.Ketones.and.Phenol.pdf (556kB)
This file has been downloaded 617 times
Sandmeyer
Nice find, it is attached. As to 'speed freaks' coming up with much at all, you've obviously not been on WD anytime lately.
Attachment: Glennon.B.Oxygenated.Analogues.of.the.5HT2A.Serotonin.Receptor.Agonist.DOB.pdf (117kB)
This file has been downloaded 763 times
Back to the Subject
I adore the level of negativity, it always amuses me... All I've done is ask about a short-route to 2CX (using non-explosive chemicals that aren't hot
as fuck). Shit, I should be drawn & quartered.
The reason I was thinking about the whole thing was the sheer number of newish-ligands, with µg/doseages based thereon
Attachment: Nichols.etal.High.Specific.Activity.Tritium.Labeled.N.2.MethoxyBenzyl.25.Dimethoxy.4.Iodophenethylamine.INBMeO.A.High.Af (245kB)
This file has been downloaded 885 times
Attachment: Braden.etal.Molecular.Interaction.of.Serotonin.5HT2A.Receptor.Residues.Phe339.and.Phe340.with.Superpotent.N.Benzyl.PEA.A (4kB)
This file has been downloaded 597 times
As to the 1-halo-2,5-dimethoxybenzene, does anyone see any real difficulty in reaching them via the nitration of acetaminophen (which reportedly gives up to 87% yield (via N-acetyl-p-benzoquinone imine) when H2O2 is used in addition to HCl & NaNO2 on acetamidophen
- whereas the 4-hydroxyaniline (from the hydrolysis of acetamidophen) should give the 1-Nitro-2-Hydroxy-4-phenylhydrazide in one pot - hydrolysis of
that should give the 1-nitro-2,5-hydroquinone shouldn't it?)
That would give us our hydroquinone and the Nitro-group ready for replacement with Bromine/Chlorine/Iodine/or even left as a Nitro group. All from
EXTREMELY OTC chemicals (if you cannot find Calcium Nitrite by now, you probably should give up). As the N-(2-MethoxyBenzyl)-2-CC &
N-(2-MethoxyBenzyl)-2CN are the best of the ligands found to date, that should be a major improvement (if it works).
Here is a Thesis (Maria Silva) on the 5-HT2A Receptors, it is a good read.
[Edited on 15-7-2010 by un0me2]
|
|
|
un0me2
aliced25 sock puppet
  
Posts: 205
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
Excuse the double-post please, but I suspect I've hit the upper limit of what I can attach to a single post. Here are Articles I-V of amines related
to 2,5-dimethoxyphenethylamine (Baltzy, Buck, et al). Some of these are prepared via the phenacylamine route, so I thought I'd throw them up.
Attachment: Amines.Related.to.2.5.Dimethoxyphenethylamine.1.pdf (408kB)
This file has been downloaded 690 times
Attachment: Amines Related to 2,5-Dimethoxyphenethylamine.1 II-1.pdf (416kB)
This file has been downloaded 632 times
Attachment: Amines Related to 2,5-Dimethoxyphenethylamine. III1 2-Hydroxy and 2-Methoxy-5-methylphenylakanolamines-1.pdf (577kB)
This file has been downloaded 2090 times
Attachment: Amines.Related.to.2.5.Dimethoxyphenethylamines.IV.25Diethoxide.2Hydroxy5Methoxy.2Hydroxy.5.Ethoxyphenethylamines.pdf (455kB)
This file has been downloaded 748 times
Attachment: Amines Related to 2,5-Dimethoxyphenethylamine. V. 2,5-Dihydroxy and 2-Methoxy-5-hydroxy Derivatives.pdf (372kB)
This file has been downloaded 679 times
As for the reduction of the aminoketone, there are a number of papers on the reduction of secondary benzylic alcohols and even ephedrine itself to the desoxy-variant using refluxing alcohols and Raney Nickel.
There are also no shortage of papers on the reduction of aminoketones (including these VERY ones) using 10%Pd/C & 55psig H2 directly to the
desoxy-PEA's/Amphetamines.
N-formylation of the amine, then a Leuckart with 2-methoxybenzaldehyde (o-methoxysalicaldehyde) should be the shortest possible route to the
super-5HT2A-Receptor Ligands.
|
|
|
|