FrankMartin
Hazard to Self
 
Posts: 50
Registered: 30-5-2010
Member Is Offline
Mood: No Mood
|
|
TLC help needed.
Can someone help me with a suitable solvent system for the analysis of products from the malonic-ester synthesis?
Also, what sort of plate coating is suitable?
The reactions involve the mono & di alkylations fom methyl to hexyl.
Thanks
Frank.
|
|
|
ziqquratu
Hazard to Others
  
Posts: 385
Registered: 15-11-2002
Member Is Offline
Mood: No Mood
|
|
Silica plates should work fine. For those compounds, I'd suspect hexane with either diethyl ether or ethyl acetate (whatever you have on hand) should
work fairly well.
I'd start with 3:1 hexane/ether (or about 9:1 hexane/EtOAc), and see how it goes. You can adjust the polarity from there once you see how your
compounds run.
Personally, I keep a few bottles of pre-mixed solvent which are appropriate to most of the compounds I work with (3:1 hexane/ether and 3:1
hexane/EtOAc being the main ones). Usually, I run the sample once with one of those, then adjust the solvent composition as required from the
information that gives me. Only exceptions are if I have been provided with a solvent system, or if I KNOW that my normal systems are going to be too
polar/not polar enough.
|
|
|
FrankMartin
Hazard to Self
 
Posts: 50
Registered: 30-5-2010
Member Is Offline
Mood: No Mood
|
|
Thanks. I have been searching Merck and others for supply of plates and they're very expensive. I will try making my own from microscope slides and
a silica slurry. I suppose the slurry can be made with hexane? Will I need some sort of binder to adhere the silica to the plate?
Frank
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
There's been other discussions on making LC plates, and I believe references were given for detailed instructions. If the site's search function
doesn't find them for you, use Google's avanced search restricted to this site,m or just do a Web search on 'making tlc plates'.
|
|
|
FrankMartin
Hazard to Self
 
Posts: 50
Registered: 30-5-2010
Member Is Offline
Mood: No Mood
|
|
YouTube has some demos on TLC
http://www.youtube.com/watch?v=EUn2skAAjHk
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Those MIT lectures are incredible! I've seen them before and still watch them.
|
|
|
FrankMartin
Hazard to Self
 
Posts: 50
Registered: 30-5-2010
Member Is Offline
Mood: No Mood
|
|
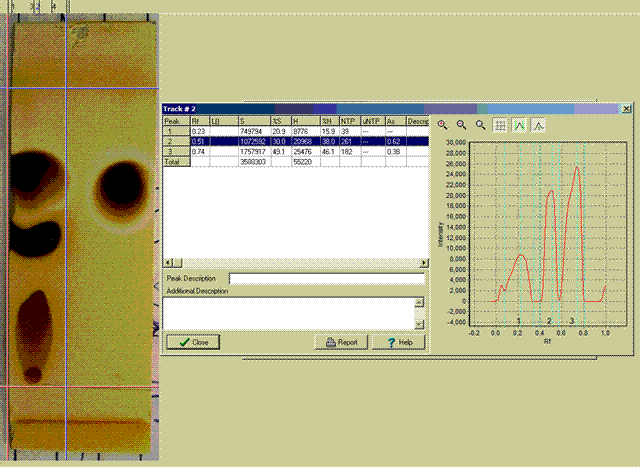
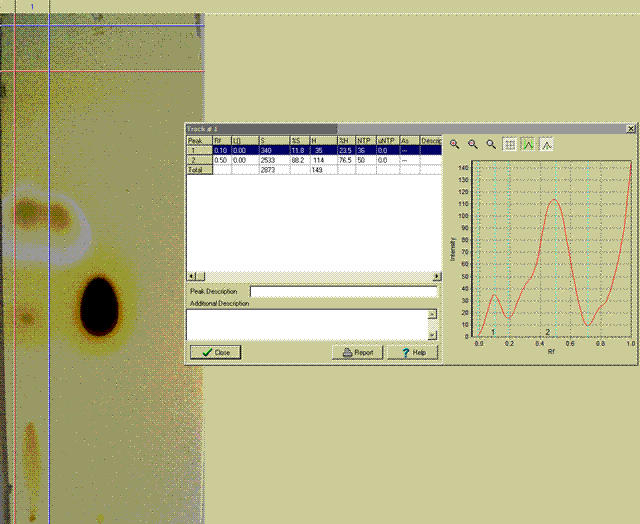
ziqquratu:
I have done as you suggested and used silca plates and a solvent mix of 25%tBuMeEther and 75% nHeptane to run them. I visualized the result with
iodine vapour. 254nm UV did not work.
The first trial was a prep of nButyl Malonic Ester (LHS) cf a sample of the Merck pure material (RHS).
The Second trial used smaller samples spotted on the plates.
Included are pictures, including the use of a trial edition densitometer program into which the plates images were just up-loaded digital photos.
I am new to all this and I need help in finding a simple inexpensive densitometer program for routine results. The one shown is very expensive.
Regards, Frank
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Please re-post the pictures. They didn't show up in your post. I2 seems a very versatile visualization medium doesn't it? I use it for esters and
amines. I get most of the spots.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 860
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
Robert Bruce Thompson has made a nice youtube video about making your own TLC silica plates: http://www.youtube.com/watch?v=pNDQkM3jasA
Yeah, it's just too bad that the woman lecturing chemistry 5.301 has the most horrible, nagging, nasal, whining voice of all the MIT employees. 
I recommend physics 1.01-1.03 with Walter Lewin. Mr. Lewin is without doubt one of the greatest pedagogic minds of the 20th century. 
|
|
|
FrankMartin
Hazard to Self
 
Posts: 50
Registered: 30-5-2010
Member Is Offline
Mood: No Mood
|
|
Reposted pictures of TLC plates.
I upload the pictures (TLC plates) relevant to my post of the 9Oct10
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
You can run semi-usable plates using proper lab filter papers if the TLCs are too much.
Filter papers are great for looking at solvents prior to using the plates. You can simply cut lots of strips, put a blob of the result on it, even
raw, and then dunk each and let it run. If you're getting careful, a dilute spotting solution is the way to go.
The resolution isn't as good, as the fibres are quite big and randomly dispersed, so you're more likely to get some streaking or blooming of the
spots. But, on developing them, the papers will give you a rough idea how it's responding to the different solvents and which might be a good idea.
Having the results a bit blurry will also encourage you to pick a solvent or pair that will produce a very fine split on the TLCs.
What's that software you're using to do the Rf's?
|
|
|
FrankMartin
Hazard to Self
 
Posts: 50
Registered: 30-5-2010
Member Is Offline
Mood: No Mood
|
|
TLC Software.
The one pictured is from the following site.
http://www.sorbfil.com/en/index.htm
and it seems OK, but they want about 400Euros for it after the 30-day evaluation period.
I am trying out some freeware at:
http://lazarsoftware.com/
but I am still trying this.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
400 Euros! 
It's handy, but a ruler does something pretty similar from the looks of it.
|
|
|
brubei
Hazard to Others
  
Posts: 188
Registered: 8-3-2015
Location: France
Member Is Offline
Mood: No Mood
|
|
Another free usefull software : gelanalyzer
http://gelanalyzer.com/download.html
|
|
|
Harristotle
Hazard to Others
  
Posts: 138
Registered: 30-10-2011
Location: Tinkerville
Member Is Offline
Mood: I tink therefore I am
|
|
TLC visualizer
NIH Image, or its java port, ImageJ.
Just the best for gel, tlc and densitometry, in my opinion, and free.
Cheers,
H
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
How do you use these pieces of software. Do you scan the plate on an ordinary flatbed scanner or photograph it (under UV light if you can) with a
digital camera or something and then open the image in the software and highlight the area to analyse? Even just the densitometry facility would be
useful.
@Harristotle. Do you have a bit more info on the TLC visualizer like a partial web address. I tried running "TLC visualizer" etc through google but I
mostly about various bits of hardware by CAMAG.
I found the gel analyser software though. Does anyone have any experience of it?
|
|
|
Harristotle
Hazard to Others
  
Posts: 138
Registered: 30-10-2011
Location: Tinkerville
Member Is Offline
Mood: I tink therefore I am
|
|
Using ImageJ for densitometry (make your TLC semi-quantitative):
http://lukemiller.org/index.php/2010/11/analyzing-gels-and-w...
or if you prefer a video tutorial, https://www.youtube.com/watch?v=JlR5v-DsTds
(note that you can just photograph with a webcam, and feed that to ImageJ).
To get a chromatograph- style analysis (allows you to calc rf easily)
https://www.google.com.au/url?sa=t&rct=j&q=&esrc...
I used to love NIH Image, back in the day .....
Fantastic for citizen science and school level projects.
Cheers,
H.
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@Harristotle, thank you very much for this. I will have a look at this.
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
This thread has reminded me of a project I looked into some years ago to make spot tests at least semi-quantitative using a scanner to scan them and
then use some kind of densitometry software to compare the test spot with standard ones. I got as far a buying the microlitre size automatic pipettes
and making up a large number of standard solutions but I couldn't find any suitable software. I vaguely remembered seeing a tool in a piece of image
processing software like Adobe or Photopaint but I can't remember where or exactly what it was designed for but thinking at the time it would do this
job. The piece of software above works with monochome images 8 bit TIFFs which would be OK for single component spots where optical density is OK but
for multi element spots or chromatographs you need to recognise the colour shift too. Any one out there got any suggestion.
|
|
|
Harristotle
Hazard to Others
  
Posts: 138
Registered: 30-10-2011
Location: Tinkerville
Member Is Offline
Mood: I tink therefore I am
|
|
Check out the plugins:
https://imagej.nih.gov/ij/plugins/
particularly RGB Measure might help you.
I am fairly sure that most lab imaging applications have been done. This was what many commercial bits of software developed from: being open source
and govt meant that it was a very good place to work out the math before commercialising.
Other plugins may also assist.
Cheers,
H.
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
Is manual analysis so hard? Usually errors are introduced during the chromatography itself, not during its analysis. You can use a simple straightedge
or any other measuring tool. Few dozens of marks might be hard to read carefully, but one or two spots are not a problem at all, unless I'm stupid and
don't understand something.
|
|
|