| Pages:
1
..
12
13
14
15
16
..
25 |
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Hydrazine by Anhydrous Chloramine & Ammonia Reaction
The following supposition must be regarded as prospective research into unknown
but plausible investigation rather than elaboration of tried and tested preparations.
Direct reaction between Chloramine and Ammonia at the moment they are formed
is possible in a deep eutectic ionic liquid made of 1 mol of Choline Chloride with
2 mols of Ammonium Sulfate as a participatory solvent. 1 mol of Calcium Hypochlorite
( HTH is 65 - 70 % available Chlorine the balance is NaCl ) , and 1 mol of Lime ( CaO )
is then added in , rapidly mixing with a blender for around 5 seconds and left to stand
inside an air tight container. After a brief period of effervescence the expected result
should yield hydrated Calcium Sulfate and Hydrazine Hydrochloride.
(NH4)2SO4 + { Ca(ClO)2 + NaCl } => { NaCl } + CaSO4 • 2H2O + 2
H2NCl
HOCH2CH2(CH3)3NCl ( Choline Chloride ) => no reaction
(NH4)2SO4 + CaO => CaSO4 • H2O + 2 NH3
_______________
2 NH3 + 2 H2NCl => 2 H2N.NH2 • HCl
The occurrence of possible side reactions is limited by the minimal duration
of the sequence of events. Temperature , pH and trace presence of catalytic
metals promoting decomposition and dissociation, remain the antagonists of
the desired path. The reaction begins in a liquid state and may remain this
way ( given that hydrogen bonding sites prevail throughout providing miscibility
with Choline Chloride ). Without a means to absorb the heat released by the
concentrated reactants ( perhaps with an admixed inert salt ) highly vigorous
even violent activity can result from the quantity of gaseous formation. Given
the reactive nature of the precursors let alone the product , the yield cannot
be quantitative.
In a related process , according to :
Syntheses of Some N-substituted Hydrazines by the Anhydrous Chloramine Process
http://www.sciencemadness.org/talk/files.php?pid=131996&...
- Quote from pdf page 7 _
" Temperature of reactor wall does not seem to have any effect on the
Chloramine yield in the range 25 to 95 ºC, although Sisler et al (1954)
observed considerable decomposition of Chloramine when the reactor
walls are kept below the ambient temperature in the range 10 to - 30 ºC "
( my comment - counterintuitively contrary to what one would expect given
that chloramine is increasingly less stable with increasing temperature )
Dissolving the product in Methanol will precipitate the Ca(SO4) • (x)H2O , NaCl.
Filtering , then adding Chloroform to the solution should acquire a precipitated
Hydrazine Hydrochloride salt.
Choline Chloride - CAS 67-48-1
Physical Form: Crystalline
Color: White
Odor: Odorless
Molecular Weight: 139.62
Boiling Point: Decomposes > 302 - 305 ºC
Melting Point: 247 ºC ,
Water solubility: 650 g/L (20 ºC)
Soluble in , Alcohol , Chloroform , and Acetone.
Insoluble in Ether and Benzene
Acidic organic salt. Solution pH 6.5 - 8 ,
reacts as acid to neutralize bases.
http://www.inchem.org/documents/sids/sids/67481.pdf
Hydrazine Hydrochloride - CAS 2644-70-4
Chemical Formula: H2N.NH2 • HCl
Physical Form: Crystalline
Color: White
Odor: Odorless
Molecular Weight: 68.51
Boiling Point: Decomposes 240 °C
Melting Point: 89-93 ºC
Solubility In Water: 370 g/L (20 ºC)
See page 120 - Hydrazine Chemistry.pdf , attached below
Hydrazine Dihydrochloride - CAS 5341-61-7
Chemical Formula: H2N.NH2 • 2HCl
Physical Form: Crystalline
Color: White
Odor: Odorless
Molecular Weight: 104.97
Boiling Point: Decomposes 240°C
Melting Point: 198°C
Solubility In Water: Soluble
Specific Gravity: 1.42
Hydrazine - CAS 302-01-2
Physical Form: colorless, oily liquid, fuming in air.
Color: Colorless
Odor: penetrating odor resembling that of ammonia
Molecular Weight: 32.05
Density: 1.0036 (25/4°C)
Boiling point: 113.5°C (at 760 mm Hg)
Melting Point: 1.4 - 1.5°C
Solubility: soluble in Water, Alcohol, and Isobutanol;
Insoluble in Ether and Chloroform
Vapor density: 1.04 (air = 1)
vapor pressure: 14.4 mm Hg at 25°C
Explosive limits: 4.7 - 100% by volume in air
Flash point: 38 - 52°C (open cup)
Fire Hazard
It is a flammable/combustible material and may be ignited by heat, sparks, or flames.
Vapor may travel to a source of ignition and flash back. Container may explode in
heat of fire. Vapor explosion and poison hazard indoors, outdoors, or in sewers.
Runoff to sewer may create fire or explosion hazard. Vapors form explosive mixtures
with air. May continue to burn in the absence of air. Can catch fire when in contact
with porous materials such as wood, asbestos, cloth, earth, and rusty metals.
Incompatible with oxidizers, hydrogen peroxide, nitric acid, metal oxides, and strong acids.
Health Hazard
Organs affected include central nervous system; respiratory system; skin and eyes.
Chronic exposure in humans may cause pneumonia, liver and kidney damage. Liver
damage may be more severe than kidney damage. It is carcinogenic.
http://www.atsdr.cdc.gov/tfacts100.pdf
.
Attachment: Hydrazine Chemistry.pdf (709kB)
This file has been downloaded 1325 times
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Another possible hypothetical reaction scheme
The classic deep eutectic ionic liquid made of 1 mol of Choline Chloride with 2 mols of
Urea as a participatory solvent. 1 mol of Calcium Hypochlorite ( HTH is 65 - 70 %
available Chlorine the balance is NaCl ) , and 1 mol of Calcium Hydroxide ( Ca(OH)2 )
is then added in , rapidly mixing with a blender for around 5 seconds and left to stand.
After a brief period of effervescence the expected result should yield Calcium Carbonate
Calcium Chloride monohydrate, CO2 and anhydrous Hydrazine.
Vigorous foaming should be expected with the additional formation of Carbon dioxide.
(NH2)2CO + { Ca(ClO)2 + NaCl } => { NaCl } + CaCO3 + 2 H2NCl
HOCH2CH2(CH3)3NCl ( Choline Chloride ) => no reaction
(NH2)2CO + Ca(OH) => CaCO3 + 2 NH3
_______________
2 NH3 + 2 H2NCl => 2 H2N.NH2 •HCl
2 H2N.NH2 •HCl + CaCO3 => CaCl2 •H2O + CO2 + 2
H2N.NH2
Dissolving the product in Methanol will precipitate the CaCl2 •H2O and CaCO3. Filtering ,
then adding Chloroform to the solution should partition an upper Hydrazine phase.
[Edited on 28-3-2010 by franklyn]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Yet another reaction scheme
Direct reaction between Chloramine and Ammonia at the moment they are formed
is possible in a deep eutectic ionic liquid as a participatory solvent made of 1.5 mol
of Choline Chloride with 3 mols of CaCl2 •6NH3 ( produced beforehand by introducing
ammonia into a sealed container holding anhydrous Calcium Chloride ).
2 mols of Trichloro-isocyanuric acid is then added into this , rapidly mixing with a blender
for around 5 seconds and left to stand inside an air tight container. After a brief period
of effervescence the expected result should yield Calcium Isocyanurate and Ammonium
Chloride and Hydrazine Hydrochloride.
The overall reaction is _
3 CaCl2 •6NH3 + 2 ( ClNCO )3 => Ca3{( NCO )3}2 + 6 NH4Cl + 6 N2H4 •HCl
HOCH2CH2(CH3)3NCl ( Choline Chloride ) => no reaction
The steps are _
6 NH3 + 2 ( ClNCO )3 => 2 ( HNCO )3 + 6 H2NCl
6 NH3 + 6 H2NCl => 6 N2H4 •HCl
3 CaCl2 + 2 ( HNCO )3 => Ca3{( NCO )3}2 + 6 HCl
6 NH3 + 6 HCl => 6 NH4Cl
____________________________
I now believe that my expectations for the results as given in the preceding two posts and
the third proposed method above are overly optimistic given that I have not accounted for
competing reaction pathways. The Chloramine as produced does not occur as it does in the
gas phase reaction of elemental Chlorine with Ammonia since Chlorine in those procedures
are not diatomic , sparing at least this initial formation of Ammonium Chloride.
2 NH3 + Cl2 => H2NCl + NH4Cl
Condensation of Chloramine with Ammonia produces Hydrazine and preferentially Ammonium
Chloride as well. Hydrazine will displace Ammonia from Ammonium Nitrate , it does not however
displace Ammonia from Ammonium Chloride , and that will not react further , sequestering it's
ammonia. As a result yield is likely to be half of what I projected unless twice the molar ratio
of reagent ammonia is provided.
2 NH3 + H2NCl => N2H4 + NH4Cl
Chloramine itself reacts with Hydrazine and is the major antagonist to the desired reaction.
H2NNH2 + 2 H2NCl => N2 + 2 NH4Cl
All indications are there is no way to produce hydrazine neat without extensive side products.
Clearly reaction kinetics largely determine the preparatory value of a prospective procedure.
.

|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
This is a huge thread for making hydrazine, and there are others. Why is it that I've only seen the Raschig method mentioned once? And there was no
discussion of its merits or of anyone trying it. It's a very straightforward procedure, taking up less than one page in Brauer (p. 468).
Brauer (Raschig) specifies 20% aqueous ammonia, 1N (7.5%) NaOCl ,and H2SO4 as reactants. 1% limewater is used to complex metal ions. The equation
presented is:
2NH3 + NaOCl + H2SO4 = N2H6SO4 + NaCl + H2O
I did note that the amount of NaOCl specified seems to be off by a factor of 10, however. Perhaps this is a typo?
Other than being able to use urea instead of 20% ammonia, which is less OTC, I'm puzzled as to why this method doesn't seem to be in use by members of
this forum. Can anyone tell me why this is?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Quote: Originally posted by Magpie  | This is a huge thread for making hydrazine, and there are others. Why is it that I've only seen the Raschig method mentioned once? And there was no
discussion of its merits or of anyone trying it. It's a very straightforward procedure, taking up less than one page in Brauer (p. 468).
Brauer (Raschig) specifies 20% aqueous ammonia, 1N (7.5%) NaOCl ,and H2SO4 as reactants. 1% limewater is used to complex metal ions. The equation
presented is:
2NH3 + NaOCl + H2SO4 = N2H6SO4 + NaCl + H2O
I did note that the amount of NaOCl specified seems to be off by a factor of 10, however. Perhaps this is a typo? |
No, it's not. A similar ratio is seen in the OrgSyn method.
| Quote: | | Other than being able to use urea instead of 20% ammonia, which is less OTC, I'm puzzled as to why this method doesn't seem to be in use by members of
this forum. Can anyone tell me why this is? |
The percent yield is quite a bit better than some procedures that use ammonia (like the OrgSyn procedure).
It also could have to do with the volume of reagent (using less urea than ammonia solution).
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thanks Formatik. I didn't even suspect that OrgSyn would have a synthesis for hydrazine sulfate. I thought that it would be commonly available from
the US chemical industry. I guess when OrgSyn was started the US was almost totally dependant on Germany for such chemicals.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Quote: Originally posted by Magpie  |
Thanks Formatik. I didn't even suspect that OrgSyn would have a synthesis for hydrazine sulfate. I thought that it would be commonly available from
the US chemical industry. I guess when OrgSyn was started the US was almost totally dependant on Germany for such chemicals. |
That's right, Magpie. See this link for the history.
|
|
|
kharof
Harmless

Posts: 1
Registered: 22-1-2010
Member Is Offline
Mood: No Mood
|
|
i found these
http://www.freepatentsonline.com/pdf_collections_server1/usp...
ammonia + acetone + hypochlorous acid --> acetazine + H2SO4 --> HS
how i can to prepare hypochlorous acid?????????????
(Cl2 + H2O ---> HOCl + HCl i tried this but how to rremove HCl)
(Ca(OCl)2 + 2H2O --> Ca(OH)2 + 2HOCl i tried this but no smell chlorine and i remmember these hypochlorous acid smell chlorine)
i tried this:
ammonia + acetone + calcium hypochlorite and after a reaction i filtered it and added
H2SO4 produced precipitate large I heated it but not vaporize (i thought calcium sulfate) but when i added it into solution from NaOH smelled
hydrazine)
HCl no thing (I thought should to precipitate CaCl2)
HNO3 (no thing)
then tried this:
ammonia + acetone + sodium hypochlorite and after a reaction a solution become yellow and evolve chlorine
then i added H2SO4 nothing
then tried this:
ammonia + acetone + H2O2 60% (i added sodium phosphate and acetamide(i not sure that is acetamide because i prepared it (i prepared it from CH3COOH +
NH3CONH3 then reflux)))
and solution become boil when I added H2O2
and I tried it:i was sure these will failing
ammonia + H2O2 + gelatin+ heated in sealed bottle and after 2 minute my bottle exploded (pressure)
i prepared HS from method NH2CONH2 + naocl but yield is small and Nh3 + naocl but yield is very bad
my result: I need help
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I thought I'd post the results of two recent syntheses of hydrazine sulfate (HS).
My first synthesis utilized the chlorourea process as first expressed by garage chemist. Then ordenblitz developed a procedure from this. I used the
ordenblitz procedure verbatim, except I substituted an equivalent amount of 12.5wt% NaOCl for Ca(OCl)2. My urea was recrystallized from urea
fertilizer, which is dirt cheap. As ordenblitz stated, this procedure progressed quickly. Yield was 5.6g, or 33% based on the amount of Cl2
generated. A melting point of 254-255C was obtained, which agrees with the Baker Chemical MSDS value of 254C.
My second synthesis used the Hofmann reaction of urea as found in the procedure of Mr Anonymous. I used a scaled down batch size of 18.7%. Although
more time consuming, the yield here was 31g, or 53% based on the amount of 12.5% NaOCl used. A picture of the HCl neutralization, which follows the
generation of the hydrazine, is shown below.
It would be interesting to know which procedure is considered safer, especially since the Mr A procedure avoids a stable chlorourea intermediate.

[Edited on 27-5-2010 by Magpie]
[Edited on 27-5-2010 by Magpie]
[Edited on 27-5-2010 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
The Mr. A procedure, which is actually an adapted Bayer patent process IIRC, is safer. It's probably the most straightforward synthesis of hydrazine
there is. You know you want to scale it up though, probably in a 12 liter flask you can use a whole gallon of pool hypochlorite in one batch.
The stuff doesn't keep well so you may as well use it all at once. Beware the bubble monster ! 
[Edited on 27-5-2010 by Rosco Bodine]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by Rosco Bodine  | The Mr. A procedure, which is actually an adapted Bayer patent process IIRC, is safer. It's probably the most straightforward synthesis of hydrazine
there is. You know you want to scale it up though, probably in a 12 liter flask you can use a whole gallon of pool hypochlorite in one batch.
The stuff doesn't keep well so you may as well use it all at once. Beware the bubble monster ! 
[Edited on 27-5-2010 by Rosco Bodine] |
Yes, the Mr A procedure is quite straightforward, and I want to thank Mr A for doing the research work to optimize it.
In regard to getting rid of nearly 1 gallon of swimming pool bleach I must say that you have a point! Maybe I should start a business of sanitizing
bathrooms, locker rooms, etc until I can get rid of it. 
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
You are welcome. I would reiterate that the Mr. A improved method is a very carefully worked out and many times tested and optimized procedure, which
is reliable and confirmed multiple times, but is also a fickle reaction sensitive to small variables. The optimized reaction conditions window is
narrow but is quite an easy target to hit if the process is followed exactly as described. Following the described process faithfully should reliably
reproduce the stated yields. Varying the described process details by even a small amount will be something like fumbling around, awkwardly trying to
find a keyhole in the dark. For all the doubters ...yeah well ....live and learn.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
I hesitate to repeat this, since it is already posted, but I feel it probably got overlooked by many so I am putting it here:
Making N2H4:
A buret slowly drips NaOCl bleach into a beaker. The beaker has two layers, a top NH4OH with some (NH4)2SO4 dissolved in it, and a bottom hydrophobic
layer resistant to being oxidized by bleach. As hydrazine is formed, it immediately precipitates out as hydrazine sulfate (3.4g/100mL 25C) which falls
into the bottom layer, where it is protected from further oxidation by the bleach. This would bypass the main difficulty of hydrazine synthesis: that
bleach attacks the hydrazine formed faster than it makes chloramine. This might allow a higher yield of hydrazine, without having to use starch or
gelatin. Ammonium sulfate solubility is 70.6g/100mL at 0C. Na2SO4 is 4.76 at 0C, not much higher than the hydrazine so you will likely get some of
this precipitating out at the bottom too.
You might also see my post: "High Yield Hydrazine Synthesis?" which presents a completely different way to make dimethyl or tetramethyl hydrazine,
where the hydrazine derivitives are protected from oxidation by bleach as soon as they are formed, so no starch or excess ammonia is required.
|
|
|
Alexein
Harmless

Posts: 35
Registered: 14-7-2007
Member Is Offline
Mood: Metastable
|
|
Quote: Originally posted by Anders Hoveland  | I hesitate to repeat this, since it is already posted, but I feel it probably got overlooked by many so I am putting it here:
Making N2H4:
A buret slowly drips NaOCl bleach into a beaker. The beaker has two layers, a top NH4OH with some (NH4)2SO4 dissolved in it, and a bottom hydrophobic
layer resistant to being oxidized by bleach. As hydrazine is formed, it immediately precipitates out as hydrazine sulfate (3.4g/100mL 25C) which falls
into the bottom layer, where it is protected from further oxidation by the bleach. This would bypass the main difficulty of hydrazine synthesis: that
bleach attacks the hydrazine formed faster than it makes chloramine. This might allow a higher yield of hydrazine, without having to use starch or
gelatin. Ammonium sulfate solubility is 70.6g/100mL at 0C. Na2SO4 is 4.76 at 0C, not much higher than the hydrazine so you will likely get some of
this precipitating out at the bottom too.
You might also see my post: "High Yield Hydrazine Synthesis?" which presents a completely different way to make dimethyl or tetramethyl hydrazine,
where the hydrazine derivitives are protected from oxidation by bleach as soon as they are formed, so no starch or excess ammonia is required.
|
I don't think it would work, but i've been wrong before. Please post yields and pictures of your results, as well as characterization or confirmation
experiments. I think silver nitrate works well to test hydrazine.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Good not to confuse speculation with knowledge. Theory is just that. It has its usefulness for indication of what may be interesting basis for
experiments to confirm or disprove what is predicted by theory. But one can get lost in theoretical ramblings which escalate to building further
theory predicated upon earlier unproved theory as if it were an inconsequential missed step to establish the premise for advancing further.
Scientific method is nuts and bolts systematic work and not a fiction novel added chapter by unproven chapter. Methods tested and proven valid and
theory based proposed experiments are not to be confused. The first is a given and the second is a maybe. Sometimes the experimental and
theoretical prediction of 1 + 1 = 2 is correct and sometimes it isn't. The experiment done and results determined accurately is what tells you if
the theory was on track or off.
|
|
|
benzylchloride1
Hazard to Others
  
Posts: 299
Registered: 16-3-2007
Member Is Offline
Mood: Pushing the envelope of synthetic chemistry in one's basement
|
|
| Quote: |
Beware the bubble monster !
|
I have had several experiences with the bubble monster. I ran this reaction several times at 1/2 scale in a 3L 5-neck flask with a mechanical stirrer
several times without problem. The last two times i ran this synthesis, I lost much of the reaction mixture due to foaming upon adding the urea
solution. I consistently got 50% yields when the reaction did not foam though.
Amateur NMR spectroscopist
|
|
|
gregxy
Hazard to Others
  
Posts: 421
Registered: 26-5-2006
Member Is Offline
Mood: No Mood
|
|
Atofina PCUK cycle
Has anyone tried this method to make hydrazine?
It uses NH3, acetone and H2O2
http://en.wikipedia.org/wiki/Atofina%E2%80%93PCUK_cycle
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Industrial methods are optimized for economy of scale
and are never one pot procedures.
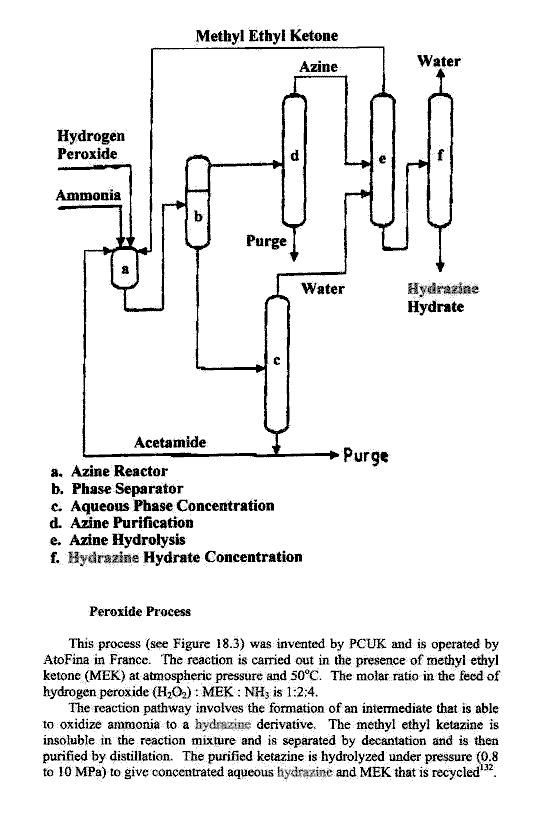
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Magpie  | This is a huge thread for making hydrazine, and there are others. Why is it that I've only seen the Raschig method mentioned once? And there was no
discussion of its merits or of anyone trying it. It's a very straightforward procedure, taking up less than one page in Brauer (p. 468).
[snip] |
A bit dated perhaps —
Audrieth and Ogg
The Chemistry of Hydrazine
John Wiley & Sons 1951
Describes the Raschig synthesis in some detail.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
This the PCKU method is cover by US Patent 3 869 541. 1975.

|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by len1  | Quote: Originally posted by benzylchloride1  | | I ran the hydrazine sulfate synthesis described on Sciencemadness that uses 10% NaOCl solution and urea, last year. This synthesis worked well and I
obtained 185g of hydrazine sulfate. I am about to run the synthesis on a 1/2 scale in a 3 liter flask with mechanical stirring. The foaming problem
seems to be the only issue with this synthesis; the first batch I attempted overflowed the reaction flask. At least it was in a fume hood, so the
clean up was fairly easy. This was with the full scale procedure in the 3L RBF. I had the plastic funnel attached to the flask as stated. The second
attempt was conducted in a 5 gallon plastic bucket and transferred to the 3L RBF after the foaming stage via a syphon. I obtained a good yield of
hydrazine sulfate after neutralization, 185g. I am trying to see if this reaction could be scaled down without severly decreasing the yield. I
synthesised 2,4 dinitrophenyl hydrazine, and 3,5-dimethyl pyrazole using some of the hydrazine sulfate. I plan to synthesize some luminol using
readily available chemicals, including the hydrazine sulfate produced via this reaction. |
The trouble with that synthesis, is that despite a great deal of detail its rather imprecise in its key point. For instance the volume of bleach is
measured with great precision 1892ml, but then the bleach strength is quoted at 10%. Just a 1% change to 11% bleach strength alters the water content
by 180ml, which makes a mockery not only of measuring the volume to that precision, but also the subsequent attempts to dissolve the NaOH in bleach,
and the urea, and gelatine in a barely adequate amount of water.
There is the possibility that what seems to be a round figure of 10% in reality is accurately measured. That is unlikely, as the author does not seem
to have performed bleach titrations. For instance he states that 10% bleach solutions decompose within a few weeks, to a couple of months. I have
done bleach titrations, and a solution 11.5% strong, is still 8.2% even after 3 months non air-conditioned storage in temperatures which have exceeded
40 degrees for long periods of time. |
Actually a 1% variance would equate more closely with 18ml of H2O than would it equate with 180ml of H2O, since the hundredth part (1%) of 1892ml is
not the tenth part as your misplaced decimal point would indicate. You wouldn't want to be one to make a mockery of precision so let me help you stay
straight on your arithmetic.
I have several very accurate instruments which I use when conducting the experiments which I sometimes do. If I state that I used a particular amount
of a standard commercial item labeled and designated as a particular strength, then I stated the measured quantity accurately in all probability,
although I am human and I may have inadvertantly stated 1892ml instead of 1900ml. However, it is more probable that if I stated 1892ml, then it was
measured in a graduated cylinder of at least reasonable quality like Kimax or Pyrex class A glassware which would be accurate to plus or minus 2ml, so
it may have actually been 1890ml or 1894ml which was used.
Not doing a bleach titration in order to satisfy your desire for greater precision should not be qualified as making the reported synthesis
troublesome. Anyone is welcome to refine the details and embellish the process any way they wish, including doing bleach titrations if they think
that is necessary to validate or make the work complete.
When doing bleach titrations, try to keep your decimal point in the right place and that way you will know better within plus or minus a power of ten
what your bleach titration has revealed. Then you can be better equipped to quibble about what a difference one per cent would make.
On page 8 of this thread you will find the same file posted concerning the commercial bleach product and its analysis,
and although I did not do a chemical analysis of the bleach, I most definitely did check its density to have a reasonable certainty of what I was
talking about and stating as accurately as possible here.
Attachment: bleach.pdf (80kB)
This file has been downloaded 1036 times
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
Another day, another smart alec argument on SM. Why dont some of you guys get more interested in science than trying to score senseless points over
each other while contributing nothing scientifically.
It is clear you are just clamoring an argument since I did state a change of 1% to 11%. Not by 1%. Its an additive change, and % is the appropriate
unit. The statement is perfectly clear and there is nothing wrong with it.
Scientifically its also valid, since bleach, as stated in one of the posts can change substantally in concentration over a matter of weaks. Commercial
bleach is not quoted to 1% accuracy, nor should it be due to the time variability. Hence an additive change of 1% is much more reasonable to consider
than a fractional 1%. So the point is also valid scientifically.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
There is no desire by me to be a smartass or argue. It simply raised my ire a bit over the indignity that you would think or propose by inference
that I am a careless technician who would not show ordinary diligence about understanding or justifying my numbers. I am not an incompetent or
careless technician. So generally if I state a number, then it is reasonably accurate and reliable. I do try to contribute scientifically and that
pursuit is not a game for me. If what I report is inaccurate and does not withstand scrutiny then I will assuredly correct any error when I learn of
it.
http://www.youtube.com/watch?v=97WlNx-vJGs&fmt=18 Spiritual Vitamins
http://www.youtube.com/watch?v=ZEbeMvaVs68&fmt=18 You're All I Need to Get By
You're all I need to get by.
Like sweet morning dew, I took one look at you,
And it was plain to see, you were my destiny.
With arms open wide, I threw away my pride
I'll sacrifice for you, Dedicate my life to you
I will go where you lead, Always there in time of need
And when I lose my will
You'll be there to push me up the hill
There's no, no looking back for us
We got love sure 'nough, that's enough
You're all, You're All I need to get by.
You're all I need to get by.
Like an eagle protects his nest, for you I'll do my best,
Stand by you like a tree, dare anybody to try and move me
Darling in you I found
Strength where I was torn down
Don't know what's in store but together we can open any door
Just to do what's good for you and inspire you a little higher
I know you can make a man out of a soul that didn't have a goal
Cause we, we got the right foundation and with love and determination
You're all, you're all I want to strive for and do a little more
You're all, all the joys under the sun wrapped up into one
You're all, You're all I need to get by.
[Edited on 18-8-2010 by Rosco Bodine]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Attachment: Hydrazine Sulfate preparation from Practical Inorganic Chemistry - Vorobyova.pdf (101kB)
This file has been downloaded 1218 times
Excerpted from Practical Inorganic Chemistry by Vorobyova.O.I., Dunaeva.K.M., Ippolitova.E.A., Tamm.N.S.
Cited here - http://www.sciencemadness.org/talk/viewthread.php?tid=9824&a...
relates to the preparation cited earlier in this thread by YeOldeImpurities
http://www.sciencemadness.org/talk/viewthread.php?tid=1128&a...
.
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
No I did not mean it like that. Everyones human. I make errors like that. Doesnt mean anything characterwise. And whats the point of me posting if i
dont get criticism. it just has to be objective chriticism then its fine. People dont understand here, undeserved flattery is as good as condemnation.
This was posted a while back. I actually did the experiment to see how it compared to my previous method. Got an explosion because my hypochlorite
was 12%. hence the post
|
|
|
| Pages:
1
..
12
13
14
15
16
..
25 |