| Pages:
1
2 |
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Horribly Dangerous, but useful, Reaction
I bubbled acetylene into a solution of AgClO4.
Ag2C2 precipitated out at the bottom, and presumably, HClO4 formed in solution. Want to get a hold of some benzene to try absorbing AgClO4 from
solution in water, to the benzene. Then bubbling H2C2 into the AgClO4 in benzene to obtain pure HClO4 which should separate into a separate layer.
Interestingly AgClO4 is quite soluble in benzene. This is obviously dangerous because Ag2C2 is somewhat sensitive, and could trigger an explosion with
the HClO4.
|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
This reaction is not exactly useful. Since synthesizing any large amount of perchlolric acid would be REALLY expensive. Silver perchlorate is much
more expensive than perchloric acid itself. The only really expensive thing about perchloric acid is hazmat shipping.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
But there are not too many ways for the basement chemist to obtain anhydrous HClO4. The Ag2C2 is a nice byproduct too.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Q: What do you call someone who prepares a mixture of silver carbide, anhydrous perchloric acid, and an organic solvent?
A: Deceased.
|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
Anders Hoveland,
Perchloric acid is available to the home chemist, I have found two companies who will ship to individuals. You just need to look around a little bit.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Online
Mood: interested
|
|
Yes, it is available to home chemists. I have some of it (a 60% solution, which can be safely handled without fear of explosions).
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
 Anhydrous Perchloric Acid? Anhydrous Perchloric Acid?  There is a reason why it's sold at levels up to 70% and no further. The anhydrous product would (in all likelihood) need to be shielded
from all fuels. There is a reason why it's sold at levels up to 70% and no further. The anhydrous product would (in all likelihood) need to be shielded
from all fuels.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
I am unsure about this. Any knowledgeable comments would be welcome. I think even anhydrous HClO4 is not a reactive oxidizer at room temperature. Sure
it is so sensitive it will explode with almost anything, but that explosion is triggered by the hydrolysis of a strong acid, not the acid oxidizing
anything. Like H2SO4, it must be heated to dissolve lead. I am almost certain that the acid anyhydride, Cl2O7, will dissolve without reaction in
benzene.
I know dilute HClO4 is not even reduced on reaction with zinc, while liberating hydrogen, whereas HNO3 would give off nitric oxide.
[Edited on 23-6-2010 by Anders Hoveland]
|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
No no,
Anhydrous and even azeotropic perchloric acid are very powerful and dangerous oxidizers. There are many different reports of fires and explosion
resulting from perchloric acid solutions coming into contact with common organics like sawdust and paper. Certainly anhydrous HClO4 in benzene with
silver carbide would be a sensitive and deadly explosive. Sure perchloric acid may not react with benzene, but the resulting mixture would be very
easy to detonate, especially with a primary explosive floating around in it.
If you want some actual accounts of perchloric acid fires/explosions, there are reports all over the net. Or you could ask the Wizardisin he seems to
know quite a few stories about things like this.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
(sigh)
Read what follows. Also go talk to a lawyer and have them explain the legal term endangerment to you, slowly and clearly
and making sure you understand it. And then think long and hard about posting any more ideas that you haven't actually tried yourself, or have links
to papers where their were performed.
http://safety.dri.edu/Hazards/PerchloricAcidGuidelines.pdf
http://www.safety.seas.harvard.edu/advise/PerchloricAcid.htm...
Anhydrous perchloric acid (> 85% concentration) is very unstable and will usually explode when it comes in contact with organic materials. Follow
these additional precautions when working with anhydrous perchloric acid.
· Allow only experienced research workers to handle anhydrous perchloric acid. These workers shall be thoroughly familiar with the literature on the
acid. Assure that a second worker is informed of the intended use of the anhydrous perchloric acid. This second worker should be in sound or sight
contact with the worker using anhydrous perchloric acid.
· Use a safety shield to protect oneself against the effects of a possible explosion.
· Use the acid in a designated, properly designed perchloric acid hood with a minimum of equipment present. No extraneous chemicals should be present
in the hood.
· Use thick gauntlets in addition to PPE previously recommended.
· Use only freshly prepared acid. Do not make any more anhydrous perchloric acid than is required for a day/shift.
http://ehs.uky.edu/ohs/perchloric.htm
Perchloric acid should be stored separately from many other compounds including acetic acid, acetic anhydride, alcohols, aniline, bismuth and bismuth
alloys, combustible materials, dehydrating agents, ethyl benzene, hydriotic acid, hydrochloric acid, grease, iodides, ketones, other organic
materials, oxidizers and pyridine.
from a MSDS
Special Remarks on Explosion Hazards:
Decomposes when distilled at atmospheric pressure, sometimes with explosive violence. Undergoes spontaneous and explosive decomposition.
Perchloric acid + acetic acid can cause explosion. Hot concentrated perchloric acid acid + alcohols or cellulose is particularly dangerous. Perchloric
acid + aniline and then formaldehyde gives resinous condensation product which burns with explosive violence. Drop of anhydrous perchloric acid
+diethyl ether causes explosion. Drop of anhydrous perchloric acid on charcoal causes explosion. 70% Perchloric acid solution reacts instantly and
explosively on contact with Dibutyl Sulfoxide. Perchloric acid + ethyl alcohol or methyl alcohol can cause explosion. Perchloric acid + most organic
materials can cause explosion. Drop of anhydrous perchloric acid on paper can cause violent explosion. Explosion occurs when 70% perchloric acid
contacts sulfoxides. Some inorganic materials, such as hypophosphites, tend to form explosive mixtures with perchloric acid when hot. Reaction of
anhydrous perchloric acid and wood fibers or dust causes violent explosion. Contact of fluorine and 72% perchloric acid at ambient temperature
produces a high yield of explosive gas, fluorine perchlorate. Perchloric acid + nitrogenous epoxides causes precipitaition of organic perchlorate
which is highly explosive.
From
A comprehensive guide to the hazardous properties of chemical substances by Pradyot Patnaik

Do you notice anything in common in these? The phrases "can explode" or "may explode" seem to show up a lot. Now consider these:
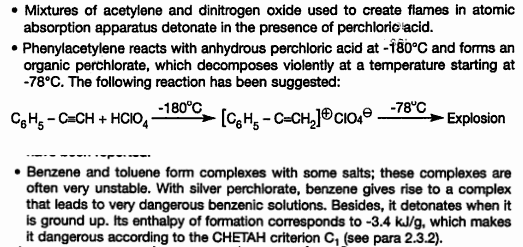
While one talks about a mix of C2H2 and NO2, and the next about phenylacetylene, information such as that should make you cautious until you have
proof - which isn't you saying "I don't think it does" - that the reactants will not blow up because of the phase of the moon.
| Quote: | | I am almost certain that the acid anyhydride, Cl2O7, will dissolve without reaction in benzene. |
How certain? Enough to perform the test yourself?
Also note that a common method of heavy metal acetylides is to treat them with a strong acid, which forms acetylene and metal salt. HClO4 is a strong
acid, what might happen if your procedure did make much anhydrous HClO4 .
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Those articles were particularly vague about the exact reactivity of concentrated HClO4. Of course it forms dangerously sensitive explosives with
anything organic, but a question unanswered is does the acid's hydrolysis with alcohals generate the heat to trigger the explosion, or does the acid
actually have the ability to directly attack hydrocarbons (alkanes) at room temperature?
A tiny drop of Cl2O7 dissolved in a liter of benzene is not going to explode. I would really like to know whether a drop of pure HClO4 will explode if
it falls into heptane.
That HClO4 would explode with a ketone or even ether is not particulary impressive. Will it automatically expode on contact with methane, without any
trigger?
[Edited on 23-6-2010 by Anders Hoveland]
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Online
Mood: interested
|
|
I also have the feeling that working with anhydrous HClO4 is insanely dangerous. I have 60% HClO4 and I have boiled some of this down to a little over
200 C, which would give 70% HClO4 (the azeotrope is somewhere between 71% and 72%).
The 60% and even the 70% solutions are amazingly inert from an oxidative point of view (of course they are very acidic). I tried adding KI to 70%
HClO4 and even when this solution is boiled then only a very pale yellow color is produced. This pale yellow color might even be due to oxidation by
oxygen from the air and not by the perchloric acid. The same is true for adding Na2SO3 to 70% acid. This results in a strong smell of SO2 and no
oxidation occurs.
Compared to 70% HNO3 the perchloric acid is very tame. When KI or Na2SO3 is added to 70% HNO3 then immediately a very violent reaction starts and
copious amounts of NO2 are produced.
But it also is known that 70% HClO4 is almost 100% ionized as H3O(+) and ClO4(-) and the resonance stabilization in the perchlorate ion is very
strong. So, as soon as covalent HClO4 (not dissolved) is present then the story changes dramatically. I could try making a tiny amount of HClO4 (by
adding H2SO4 and P4O10 as I did before in a small test tube experiment on mg quantities) and add a drop of an organic like benzene or hexane. This,
however, will have to wait till next weekend, right now I am far from home.
[Edited on 23-6-10 by woelen]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | | I would really like to know whether a drop of pure HClO4 will explode if it falls into heptane. |
Then try it, or do some real research to locate the answer, rather than saying it probably doesn't and then being a lazy PITB waiting for someone else
to do your work for you. You really need to meditate upon that term endangerment when when batting your baby blues at others in hopes they'll do your
work.
In fact, why don't you actually perform the original proposal? That would answer many questions, and in a more meaningful way than "a drop falling
into heptane" where the solvent is different and the amount much less than your original idea. Maybe live stream it, JIC it turns out you are wrong.
BTW - 2nd mention of Cl2O7 - topic drift penalty. The subject is not Cl2O7 but HClO4, unless you can post references as to how the properties in
question are identical for the two.
Try stop being such a wizard, what with the constant shifts of reasons and reactions
| Quote: | The title wizard is said to be derived from the archaic word "Wys-ars", meaning one who, at bottom, is very wise.
-- Sir Terence David John Pratchett |
|
|
|
the Z man
Harmless

Posts: 28
Registered: 13-6-2010
Member Is Offline
Mood: No Mood
|
|
I don't have myself experience with HClO4 but I've read some horror stories on the net. Things like:
-Once concentrated perchloric acid (I think standard 70%) was spilled on the floor and sawdust was used to adsorb it.After some time the container in
which the sawdust had been thrown detonated.
-Once some perchloric acid was concentrated by boiling under a fume hood. When, some time later, some technicians were working on the fume hood, it
detonated injuring them.
If you search on the net you'll find tons of stories like this. IMHO what you have done is suicidal.
The same stories by the Wizard Is In. I didn't realize they have been posted yet. Where does he find all that information?! http://www.sciencemadness.org/talk/viewthread.php?tid=13214&...
[Edited on 23-6-2010 by the Z man]
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
I too am far from home; in the Redwoods right now.
Cl2O7 should have similar reactivity, in terms of oxidizing ability, to pure HClO4; it is the acid anyhydride. A mix of Cl2O7 and HClO4 is soluble
together, even though only one of them will dissolve in an inert hydrophobic solvent. The chlorine and oxygen atoms in such a mixture are all
interchangeable, they readily move between molecules, similar to deuturium oxide mixed with light water.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
I too am far from home; in the Redwoods right now.
Cl2O7 should have similar reactivity, in terms of oxidizing ability, to pure HClO4; it is the acid anyhydride. A mix of Cl2O7 and HClO4 is soluble
together, even though only one of them will dissolve in an inert hydrophobic solvent. The chlorine and oxygen atoms in such a mixture are all
interchangeable, they readily move between molecules, similar to deuturium oxide mixed with light water.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Beware of the possible explosion of your drop of HClO4 or Cl2O7 when falling down your organic solvent...
By definition the drops will be heavier than the solvent...you might thus get an imediate detonation on surface...but if you have a tiny delay
(induction period)...it will go down well below the surface...and then the detonation will be like an underwater detonation...thus very much critical
to your container!
I remember a TV show on the BBC a few decades ago in "Open University" that was exposing the reactivity of the alkaline metals towards water...
Lithium was moderate, sodium was fun and bruning, potassium was much stronger...they went down the Mendeliev's table and the experimentator took
longer and longer spoons to take a safer distance from the aquarium...Rubidium made an immediate openwater explosion...but the Cesium was really
devastating due to a tiny induction period, it falled down to the bottom where it sticked and detonated the entire aquarium.
This was with something like 5g into a few 100 Litters of water...so pretty much the same as what you expect to do with a tiny "drop" into a litter of
fuel...but with the extra risk due to the flamability of the fuel...
Even to Woelen who is experienced chemist, I recommand great care in the choice for the reactor...avoid what is brittle or hard (glass, metal, hard
plastic)
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
This method is not useful, considering AgClO4 is way more expensive and difficult to obtain than HClO4!
Did it really though? Perchlorato-acetylide complex formation from C2H2 and HClO4-containing AgClO4 solution has been described (Nieuwland, Maguire,
J. Am. Chem. Soc 28 [1906], 1030). Ag2C2.2AgClO4.2H2O detonates fairly strongly, like Ag2C2.AgNO3 (R. Vestin, Svensk Kem. Tidskr. 66 [1954], 69).
Quote: Originally posted by Anders Hoveland  | | Sure it is so sensitive it will explode with almost anything, but that explosion is triggered by the hydrolysis of a strong acid, not the acid
oxidizing anything. Like H2SO4, it must be heated to dissolve lead. I am almost certain that the acid anyhydride, Cl2O7, will dissolve without
reaction in benzene. |
Don't you also think it's time you found some references and quit so much speculation?
Cold, dry C6H6 solubilizes Cl2O7, then soon afterwards a reaction occurs (A. Michael, Conn, Am. chem. J. 23 [1900] 446).
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
The user, "Bromic Acid", quoted some literature for me:
"Anhydrous perchloric acid can also be obtained by extraction into methylene chloride from a mixture of one part by volume 70% perchloric acid and
four parts 25% fuming sulfuric acid".
Thus we can assume that anyhydrous HClO4 is unreactive with CH2Cl2. He also says that he sees it in the literature dissolved in chloroform. It is to
be inferred that CCl4 would also be unreactive.
Benzene reacts with Cl2O7, fascinating! I could not find the article, despite numerous online searches. However this seems to contradict something
else I read. "Cl2O7 is a strong oxidizer as well as an explosive that can be set off with flame or mechanical shock, or by contact with iodine.
Nevertheless, it is less strongly oxidising than the other chlorine oxides, and does not attack sulfur, phosphorus, or paper when cold."
Holleman, Arnold F.; Wiberg, Egon (2001). Inorganic chemistry. Translated by Mary Eagleson, William Brewer. San Diego: Academic Press. p. 464. ISBN
0123526515.
^ Byrns, A. C.; Rollefson, G. K. (1934). Journal of the American Chemical Society 56: 1250–1251.
This came off of wikipedia.
So Cl2O7 reacts with benzene, but not paper or phosphorous when cold? One explanation might be that a protective layer is formed that prevents further
oxidation. P2O5 is known to form an adhesive layer on reaction with water that limits further hydration, for instance. However, failure of Cl2O7 to
react with sulfur cannot be explained by a protecting layer, since nothing could form that would not be soluble or a gas. HNO3 attacks sulfur, but not
benzene, so I have difficulty believing that Cl2O7 would react in an opposite way. Thus this is all quite confusing, and to make any sense of the
conflicting references is a logic puzzle. I will have to eventually conduct an experiment.
Formatik, could you perhaps provide more details about that paper, or give us a link.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | The user, "Bromic Acid", quoted some literature for me:
"Anhydrous perchloric acid can also be obtained by extraction into methylene chloride from a mixture of one part by volume 70% perchloric acid and
four parts 25% fuming sulfuric acid".
Thus we can assume that anyhydrous HClO4 is unreactive with CH2Cl2. He also says that he sees it in the literature dissolved in chloroform. It is to
be inferred that CCl4 would also be unreactive. |
Hmmm, I would expect CH2Cl2 to react with HClO4 (if not sooner, then later), since HClO4 seems to react with both CHCl3 and CCl4, and CH2Cl2 is less
oxidized. I would be especially careful with CH2Cl2, it should easily be at least a detonable mixture.
Namely, HClO4 is entierly miscible with CHCl3, the solution discolors after a few days to yellow, and in air sheds crystals of HClO4.H2O. Commercial
chloroform contains alcohol, which sheds a heavy, with CHCl3-insoluble extraordinarily explosive oil (Vorländer, v. Schilling, Lieb. Ann. 310 [1900]
374; Vorländer, Kaascht (Ber. 56 [1923] 1162).
For CCl4, HClO4 is insoluble in CCl4, and gives upon shaking, a green emulsion, which discolors brown after several minutes welling up under formation
of HCl and COCl2 (Vorländer, v. Schilling, Lieb. Ann. 310 [1900] 374). Preparation of solutions of Cl2O7 in CCl4 described in: F. Meyer, Keszler
(Ber. 54, [1921] 569).
So mixing something like CCl4 and HClO4 can cost one their lives if not wearing protective gear, doing under fume hood,etc. It is dangerous to
extrapolate so assuredly.
| Quote: | | Benzene reacts with Cl2O7, fascinating! I could not find the article, despite numerous online searches. However this seems to contradict something
else I read. "Cl2O7 is a strong oxidizer as well as an explosive that can be set off with flame or mechanical shock, or by contact with iodine.
Nevertheless, it is less strongly oxidising than the other chlorine oxides, and does not attack sulfur, phosphorus, or paper when cold."
|
Yes, Cl2O7 doesn't explode on contact with wood, paper or similar materials but just evaporates (Michael, Conn, ibid). They also note unreaction
towards sulfur and phosphorus pieces. HClO4 on the other hand does explode violently on contact with wood and paper, and especially charcoal (Roscoe,
Lieb. Ann. 121 [1862] 353).
| Quote: | | So Cl2O7 reacts with benzene, but not paper or phosphorous when cold? One explanation might be that a protective layer is formed that prevents
further oxidation. |
The reaction of benzene could have to deal with a more reactive intermediate forming. And the interaction of HClO4 with benzene has been described as
follows:
If one to two drops of HClO4 has 2 to 3 cm3 benzene poured over it, under heat evolution brown flakes precipitate. Mixing of equal volumes at first
forms a green emulsion, which then explodes (Vorländer, v. Schilling, Lieb. Ann. 310 [1900] 374). 1 g of HClO4 solubilizes in 5g of well-cooled
benzene under formation of a green solution, which when stored in a sealed tube, discolors increasingly shedding a carbon-containing material; after
completion of the reaction, no free acid is found anymore (Michael, Conn, Am. chem. J. 23 [1900] 444).
| Quote: | | Formatik, could you perhaps provide more details about that paper, or give us a link. |
The paper is here. The older paper touts stability of the heptoxide, but we know now it is definitely not stable.
[Edited on 24-6-2010 by Formatik]
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
From what you described, it sounds like HClO4 reacts faster with CCl4 than with CHCl3. How could that be?
So apparently the heat generated from Cl2O7 reacting with paper causes the Cl2O7 to evaporate before the heat builds up enough to cause a run away
reaction. Almost certainly then, theoretically, a cup of sulfur would explode if poured into a cup of C2O7. Perhaps with iodine, I(ClO4)3 forms, which
cannot evaporate fast enough to prevent explosion. Otherwise, how can you explain the explosion with Iodine, but not sulfur or phosphorous?
Thanks so much for clarifying the reactivity of anhydrous perchloric acid.
[Edited on 25-6-2010 by Anders Hoveland]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
I would even think the CH2Cl2 to be most reactive. But it is not so straight forward, and so I would expect a careful study of the reaction
intermediates (the type of species) and by-products formed and their rates to give an answer to this.
| Quote: | | So apparently the heat generated from Cl2O7 reacting with paper causes the Cl2O7 to evaporate before the heat builds up enough to cause a run away
reaction. Almost certainly then, theoretically, a cup of sulfur would explode if poured into a cup of C2O7. |
Well, in the paper above they had reacted cooled stick of sulfur with Cl2O7, and these did not react even after several days. HClO4 has been found to
oxidize phosphorus and sulfur to their highest oxides. Maybe the level of hydration in HClO4 changes the reaction.
| Quote: | | Perhaps with iodine, I(ClO4)3 forms, which cannot evaporate fast enough to prevent explosion. Otherwise, how can you explain the explosion with
Iodine, but not sulfur or phosphorous? |
Iodine and Cl2O7 do explode violently, but a very careful mixing has afforded a white powder that decomposes in water to HClO4 and I2O5. Because of
this difference in reaction rate, maybe the reason is that rapid addition of iodine could invoke a subsequently rapid decomposition and reduction
reaction of the Cl2O7 resulting in explosion.
| Quote: | | Thanks so much for clarifying the reactivity of anhydrous perchloric acid. |
Sure, though we expect you to come up with your own references. Good quality references are not always found through the internet, which can mean
spending the time in some libraries.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
A white powder that decomposes in water to HClO4 an I2O5 ?
Would like to see that paper.
Perhaps there were other decomposition products also?
If not, then IO2ClO4 was probably the original white powder.
IO2OClO3 would probably have significant ionization to IO2+ and ClO4-
Maybe HClO4 reacts with sulfur because the SO3 formed on oxidation can violently dehydrate more HClO4,
whereas Cl2O7 cannot be further dehydrated. Or maybe the SO3 formed stays solid because of the extreme dehydrating medium (it only takes a tiny bit of
water to turn a much larger ammount of pure solid SO3 into liquid). This might form a protective layer, as SO3 would be insoluble in Cl2O7, without
some mechanism of ionization to allow a reaction to occur. Cl2O7 + 2SO3 --> (O3ClO)2S2O7 would be the theoretic reaction to allow the SO3 to be
dissolved. HClO4, already ionized, might be able to more readily react and dissolve SO3.
HClO4 + SO3 --> HOSO3ClO3.
|
|
|
-=HeX=-
Hazard to Others
  
Posts: 109
Registered: 18-4-2008
Location: Ireland
Member Is Offline
Mood: Precipitating
|
|
Anhydrous HClO4?
Well, I aint sure if it worked but when TRYING to prepare organic perchlorates I mixed the 70% stuff with P2O5 to dessicate it. Upon mixing with the
various organic compounds it generally liked to explode violently. However, by cooling with dry ice-acetone it worked well... But still liked to blow
up.
Mixing 70% perchloric acid with a few drops petrol resulted in a fair few bangs.
So the idea outlined at the start is suicide!
Seriously. 500mg Pentaerythritol mixed with the stuff resulted in what sounded like a shotgun blast when the reaction blew up.
Dangerous? Fuck, yes.
If you give a man a match he will be warm for a moment. Set him alight and he will be warm for the rest of his life.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Okay, I just bubbled in acetylene into a AgClO4 dissolved in cold toluene ( I don't have any benzene ). The solution became noticeably warm. A
precipitate formed and it looked lighter in color than Ag2C2. No separate layer of HClO4 appeared. There was a slight odor of chlorine. Perhaps
perfluorobenzene, Perfluorooctanoic acid, or triflic acid could be used as the solvent without reacting with HClO4.
|
|
|
| Pages:
1
2 |