| Pages:
1
2 |
Methansaeuretier
Harmless

Posts: 45
Registered: 24-5-2010
Location: Europe
Member Is Offline
Mood: strongly alkaline
|
|
Ideas needed - Oxiation of acid sensitive asarone to 2,4,5-trimethoxybenzaldehyde
Hello,
I try to make 2,4,5-trimethoxybenzaldehyde via oxidation of asarone. The yields with KMno4/NaHCO3 are pretty bad (about 25 %) because it's oxidised
further to the corresponding acid and dichromate/H2SO4 gave also miniscule yields because asarone is very sensitive to acid.
I've read in a german chemistry forum that it's possible to oxidise isoeugenol to vanillin using nitrobenzene, but poorly I can't find any reference.
(http://www.chemieonline.de/forum/showthread.php?t=125945)
Is it possible to use dichromate without H2SO4?
Any ideas?
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Well, direct nitration of Asarone via Tetranitromethane, might produce the Nitropropenylbenzene, then.... refuxing with N-methylbenzylamine, might
produce the schiffs base of the benzaldehyde.....Plus a fair amount of Nitroethane as gas.
Shulgin advocates the procedure for production of the rare myristicin aldehyde.
The drawback being, that tetranitromethane, which is fairly easy to make, is quite difficult to handle. Highly explosive.
Another approach, and the preferred one, from an industrial standpoint, would be ozonolysis. Ozone cleaves propenylbenzenes to produce
benzaldehydes....With super high yields. Thereby, the common Eugenol, becomes (the rare in nature) Vaniilin.
There are multiple, though somewhat unreliable, postings on this subject elsewhere. Consult the Rhodium Archive, generously maintained by The Vaults
of Erowid. While you are visiting, Erowid would appreciate a donation.
http://www.erowid.org/archive/rhodium/
Some folks say negative things about The Vaults, but they provide a good service. What other site provides such truthful, personal, psychedelic
accounts?
http://www.erowid.org/experiences/exp.php?ID=19572
http://www.erowid.org/experiences/exp.php?ID=3598
[Edited on 21-6-2010 by zed]
[Edited on 21-6-2010 by zed]
[Edited on 21-6-2010 by zed]
[Edited on 22-6-2010 by zed]
|
|
|
Methansaeuretier
Harmless

Posts: 45
Registered: 24-5-2010
Location: Europe
Member Is Offline
Mood: strongly alkaline
|
|
Thank you
Both ways are very unpractical for me... at least the tetranitromethane-way doesn't sound like fun und the ozone-way sounds like very long reaction
times and a mess of side reactions, because you can't get enough clean ozone with an UV-C lamp or something similar and O2 does also oxidise the
aldehyde further to the corresponding acid during long reaction time.
What about Dichromates in non- or only slightly acidic, buffered or maybe weak alkali solutions?
BTW:
I found something interesting about the nitrobenzene-way:
http://www.lycaeum.org/rhodium/pdf/aromatic.aldehyde.synthes... on Page 237
| Quote: |
By treating nitrotoluenes with either a solution of alcoholic-aqueous sodium sulfide, a solution of sulfur in concentrated alcoholic-aqueous sodium
hydroxide, or fuming sulfuric acid, oxidation and reduction within the same molecule Tdl occur and produce aminobenzaldehydes and aminonaphthaldehydes
in yields up to 70 per cent (16,101, 194,263). Occasionally aldehydes are not produced and sometimes toluidines and aminobenzoic acids are formed as
the main products; hence the method is not general (271). |
Sounds very nice, but poorly I can't finde any ratios or further instructions.
[Edited on 21-6-2010 by Methansaeuretier]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
besides ozone
http://www.sciencemadness.org/talk/viewthread.php?tid=6914
with a phosphate buffer added after formation of the peracid and before the substrate.
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
How about epoxidation with your favorite reagent - I'll pick mCPBA. Then hydrolyze to the glycol with an acid - I'll choose perchloric because I know
it works fantastic. Next cleave the glycol - I'll choose periodate because I favor my life over Pb(OAc)4. Two side products may be the a
rearrangement of the epoxide to the acetophenone or benzyl ketone.
P.S. Ozone will not give you the benzoic acid - not to mention it would be easily freed of acidic side products. See the Criegee Intermediate and secondary ozonide here here. The aldehyde is liberated upon reduction of the secondary ozonide with dimethylsulfide or
thiourea. But yes, long reaction times will eventually rip the benzene ring to shreds.
I have no idea what you're getting at with "what about dichromates"
|
|
|
Methansaeuretier
Harmless

Posts: 45
Registered: 24-5-2010
Location: Europe
Member Is Offline
Mood: strongly alkaline
|
|
Quote: Originally posted by Arrhenius  | How about epoxidation with your favorite reagent - I'll pick mCPBA. Then hydrolyze to the glycol with an acid -
I'll choose perchloric because I know it works fantastic. Next cleave the glycol - I'll choose periodate because I favor my life
over Pb(OAc)4. Two side products may be the a rearrangement of the epoxide to the acetophenone or benzyl ketone.
|
Would'nt that mostly produce (in theory) phyenylacetone? All I want is the benzaldehyde, I'm not planning to make the amphetamine  forget your dirty thoughts! forget your dirty thoughts!
BTW I think in practice major product of working with these acids would be that: 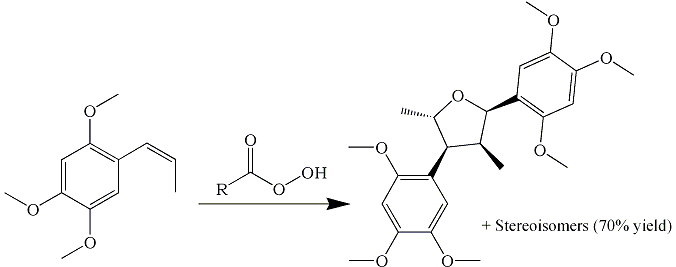
Even if you would isolate some epoxide, the rearrangement may produce similar pain. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi...
| Quote: |
P.S. Ozone will not give you the benzoic acid - not to mention it would be easily freed of acidic side products. See the Criegee Intermediate and secondary ozonide here here. The aldehyde is liberated upon reduction of the secondary ozonide with dimethylsulfide or
thiourea. But yes, long reaction times will eventually rip the benzene ring to shreds. |
Thank you. May a 1000mg/h ozone generator work for this?
[Edited on 21-6-2010 by Methansaeuretier]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Methansaeuretier  | Quote: Originally posted by Arrhenius  | How about epoxidation with your favorite reagent - I'll pick mCPBA. Then hydrolyze to the glycol with an acid -
I'll choose perchloric because I know it works fantastic. Next cleave the glycol - I'll choose periodate because I favor my life
over Pb(OAc)4. Two side products may be the a rearrangement of the epoxide to the acetophenone or benzyl ketone.
|
Would'nt that mostly produce (in theory) phyenylacetone? All I want is the benzaldehyde, I'm not planning to make the amphetamine  forget your dirty thoughts! forget your dirty thoughts! |
Oxidative cleavage of that glycol with metaperiodate gives the corresponding benzaldehyde (and not arylacetone as you seem to believe). Essentially,
what Arrhenius proposed, is to prepare the glycol in separate steps followed by the usual oxidative cleavage with NaIO4 instead of doing the oxidation
to the glycol and the cleavage to 2,4,5-trimethoxybenzaldehyde in one step like it is usually done (and requires OsO4 catalysis like in Chemistry
Letters, 32, 780-781 or WO2002079132). This way you avoid the very toxic OsO4, but the ring opening of the epoxide to the glycol is not trivial,
because acidic conditions can not be used.
By the way, have you even bothered checking the literature for the known preparations of asarone to 2,4,5-trimethoxybenzaldehyde? Somehow it appears
to me like you did not.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Methansaeuretier
Harmless

Posts: 45
Registered: 24-5-2010
Location: Europe
Member Is Offline
Mood: strongly alkaline
|
|
Quote: Originally posted by Nicodem  |
Would'nt that mostly produce (in theory) phyenylacetone? All I want is the benzaldehyde, I'm not planning to make the amphetamine  forget your dirty thoughts![/rquote] forget your dirty thoughts![/rquote]
Oxidative cleavage of that glycol with metaperiodate gives the corresponding benzaldehyde (and not arylacetone as you seem to believe). Essentially,
what Arrhenius proposed, is to prepare the glycol in separate steps followed by the usual oxidative cleavage with NaIO4 instead of doing the oxidation
to the glycol and the cleavage to 2,4,5-trimethoxybenzaldehyde in one step like it is usually done (and requires OsO4 catalysis like in Chemistry
Letters, 32, 780-781 or WO2002079132). This way you avoid the very toxic OsO4, but the ring opening of the epoxide to the glycol is not trivial,
because acidic conditions can not be used.
|
Ah ok  makes sense thank you makes sense thank you
| Quote: |
By the way, have you even bothered checking the literature for the known preparations of asarone to 2,4,5-trimethoxybenzaldehyde? Somehow it appears
to me like you did not. |
Well I don't have any literature... honestly all I was able to check was google and some e-books on my harddisk. I have no access to a libary at the
moment. All interesting I found was some bad yielding KMnO4/NaHCO3 writeups on Rhodium and of course many others with derivates of this aldehyd, but
all are working in acidic medium or with very hard obtainable reagents.
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Gotta be plans for some high yielding ozone generators hereabouts.
US Patent 2,916,499
http://www.google.com/patents?id=TQlMAAAAEBAJ&pg=PP1&...
[Edited on 22-6-2010 by zed]
[Edited on 22-6-2010 by zed]
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
I would not suggest attempting ozonolysis unless you have good laboratory techniques and proper equipment. If reductive workup is not properly
carried out, there's an excellent change you'll have an explosion. I haven't had this happen, but I have had other peroxides explode and it is not to
be taken lightly.
The preparative workarounds for this is, as reiterated by Nicodem, are osmium catalyzed cleaveage, lead(IV) acetate cleavage or periodate cleavage.
Because osmium is so incredibly toxic, it's frequently used catalytically with sodium periodate as a stoichiometric oxidant to re-oxidize osmium.
Yes, styrene oxides undergo facile rearrangement, but I have personally had a few open to the diol simply upon attempted chromatography on silica gel!
You will always find papers that will convince you not to try an experiment, but it's often better to just give it a try and see for yourself or try
to tweak things so they work for you.
This article does not mention 70% yield of anything, but it does report 15% yield of the dimer you've
drawn. Believe me, there are many many many ways you can make and isolate this particular epoxide.
| Quote: |
Well I don't have any literature..
|
Well read any published literature you can access. Read journal abstracts, supplementary information
(free online) and open access journals such as Arkivoc
| Quote: |
All interesting I found was some bad yielding KMnO4/NaHCO3
|
Honestly, permanganate oxidations are seldom used for the sole reason that they're messy and poorly
selective. Going from an alkene to a glycol with permanganate may be possible in the cold, but you'll probably have better success with other routes.
Epoxidation may also be possible using oxone. Here's a simple overview of the use of oxone to generate dimethyldioxiran (DMDO) in situ. Dioxiranes are highly effective at
epoxidation, and are non-acidic. Oxone is commonly used as a "shock" treatment for pools, as you may already know.
[Edited on 22-6-2010 by Arrhenius]
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
The above referenced patent, suggests that by ozonolysis, the target Benzaldehydes are produced directly. Without reduction.
Oxone is certainly a possibility. It's inexpensive, and easy to acquire. Yields might not be great, but its use doesn't entail the level of danger,
that using either ozone or tetranitromethane would.
|
|
|
Methansaeuretier
Harmless

Posts: 45
Registered: 24-5-2010
Location: Europe
Member Is Offline
Mood: strongly alkaline
|
|
Thank you! Sounds very promising. I'll build an ozone generator next days and I'm going to try that.
Quote: Originally posted by Arrhenius  | | I would not suggest attempting ozonolysis unless you have good laboratory techniques and proper equipment. If reductive workup is not properly
carried out, there's an excellent change you'll have an explosion. I haven't had this happen, but I have had other peroxides explode and it is not to
be taken lightly. |
I will try use cool methanol/H2O 9:1 as solvent and precipiate the product by addition of some water and strong cooling (asaraldehyde has melting
point @ 111-115 ºC. Water solubility, <0.1 g/100 mL at 22 ºC), followed by recrist. from methanol. I don't see any dangers of explosions... or am
I wrong?
| Quote: |
The preparative workarounds for this is, as reiterated by Nicodem, are osmium catalyzed cleaveage, lead(IV) acetate cleavage or periodate cleavage.
Because osmium is so incredibly toxic, it's frequently used catalytically with sodium periodate as a stoichiometric oxidant to re-oxidize osmium.
|
Sounds nice, but I don't like to work with reagents like osmium, as long as it's not neccessary.
| Quote: |
This article does not mention 70% yield of anything, but it does report 15% yield of the dimer you've drawn. Believe me, there are
many many many ways you can make and isolate this particular epoxide. |
Yes, 15% of the dimer but 70% include the other stereoisomers of the dimer. And I know that there are other ways to the epoxide, but
I don't know exactly how to get the aldehyde from the epoxide without exotic catalysts and acids.
| Quote: |
Well read any published literature you can access. Read journal abstracts, supplementary information (free online) and open access journals such as
Arkivoc |
Thank you, I didn't know Arkat. And btw. of course I read any interesting abstact and published literature I can find. But I wasn't able to find much
about oxidation of styrenes to aldehydes under acid free conditions.
| Quote: |
Epoxidation may also be possible using oxone. Here's a simple overview of the use of oxone to generate dimethyldioxiran (DMDO) in situ. Dioxiranes are highly effective at
epoxidation, and are non-acidic. Oxone is commonly used as a "shock" treatment for pools, as you may already know. |
I know, DMDO dows work very nice (90%+ yields). But Oxone is hard to get in my country.
http://www.erowid.org/archive/rhodium/chemistry/safrolepoxid...
Even CH3CN/MeOH/Na2CO3/H2O2 does also work with 40% yields after destillation:
http://www.erowid.org/archive/rhodium/chemistry/epoxide.html
Also methyltrioctylammonium phosphoperoxotungstate may work as high yielding catalyst together H2O2 as oxidant
http://www.erowid.org/archive/rhodium/chemistry/peroxotungst...
Btw, asaroneepoxide is very unstable and has to be used directly.
As said my main problem is the reaction of the epoxide to the aldehyde. Do you have a writeup or something like that for that step?
BTW: Anyone ever tested dimeric/trimeric acetone peroxide instead of DMDO for this preparation? It's very easy to produce and isolate and as long it's
kept very wet and washed neutral it's not very risky to use it. But illegal in some countries...

BTW in "Schulte-Ladbeck, R.; Kolla, P.; Karst, U. (2003). "Trace Analysis of Peroxide-Based Explosives". Analytical Chemistry 75 (4): 731–735" they
also say that under neutral conditions H2O2 and Acetone produce "monomeric aceton peroxide" very slowly and the tetrameric (much more stable) derivate
can be made if tin(IV)chloride and hydroquinone are used instead of a mineral acid. (Source: Jiang H., Chu G., Gong H., Qiao Q. (1999). "Tin Chloride
Catalysed Oxidation of Acetone with Hydrogen Peroxide to Tetrameric Acetone Peroxide". Journal of Chemical Research 28: 288–289)"
[Edited on 22-6-2010 by Methansaeuretier]
[Edited on 22-6-2010 by Methansaeuretier]
|
|
|
leu
Hazard to Others
  
Posts: 368
Registered: 13-10-2005
Member Is Offline
Mood: No Mood
|
|
The most facile route to 2,4,5 trimethoxybenzaldehyde isn't from asarone because the work up is supposedly a royal pain in the ass but through
vanillin, albeit acetic anhydride and bromine are necessary 
[Edited on 22-6-2010 by leu]
Chemistry is our Covalent Bond
|
|
|
gardenvariety
Harmless

Posts: 41
Registered: 19-1-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by leu  | The most facile route to 2,4,5 trimethoxybenzaldehyde isn't from asarone because the work up is supposedly a royal pain in the ass but through
vanillin, albeit acetic anhydride and bromine are necessary 
[Edited on 22-6-2010 by leu] |
Procedures?
Just guessing, but acetylate the phenol, brominate ortho to the aldehyde, deacetylate the phenol to make the ring active to an Ullman?
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Quote: Originally posted by gardenvariety  |
Procedures?
Just guessing, but acetylate the phenol, brominate ortho to the aldehyde, deacetylate the phenol to make the ring active to an Ullman?
|
A true gentleman would not protect/deprotect (in this case acetyl) if his desired substituent (in this case methoxy) can serve both as a directing as
well as a protecting group.
|
|
|
gardenvariety
Harmless

Posts: 41
Registered: 19-1-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sandmeyer  |
A true gentleman would not protect/deprotect (in this case acetyl) if his desired substituent (in this case methoxy) can serve both as a directing as
well as a protecting group. |
Wouldn't the unprotected phenol drive the position of the bromination? Most methods I have researched drive either para or ortho to the phenol;
brominating at the 2 is meta to the phenol. That's why I guessed acetyl; as protecting it might allow bromination at the 2 and unprotecting it would
allow for the Ullman, which is reportedly ineffective without a similarly strong EDG.
Aside from that, and aside from a meta-directing bromination, the only other route off the top of my head is methylation, oxidizing the aldehyde to
phenol, then ortho-formylation and methylation in whichever order ones' reagents allow.
That doesn't require acetic anhydride, though, which is why I'm asking leu to explain.
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Quote: Originally posted by gardenvariety  | Quote: Originally posted by Sandmeyer  |
A true gentleman would not protect/deprotect (in this case acetyl) if his desired substituent (in this case methoxy) can serve both as a directing as
well as a protecting group. |
Wouldn't the unprotected phenol drive the position of the bromination? Most methods I have researched drive either para or ortho to the phenol;
brominating at the 2 is meta to the phenol. That's why I guessed acetyl; as protecting it might allow bromination at the 2 and unprotecting it would
allow for the Ullman, which is reportedly ineffective without a similarly strong EDG.
Aside from that, and aside from a meta-directing bromination, the only other route off the top of my head is methylation, oxidizing the aldehyde to
phenol, then ortho-formylation and methylation in whichever order ones' reagents allow.
That doesn't require acetic anhydride, though, which is why I'm asking leu to explain. |
Ok. Methylation and subsequent bromination of vanillin should give you 6-bromoveratraldehyde, this can be Ullmanized...
edit:
| Quote: |
That's why I guessed acetyl; as protecting it might allow bromination at the 2 and unprotecting it would allow for the Ullman, which is reportedly
ineffective without a similarly strong EDG. |
Reportedly? Could you please post the ref./method? I have a french paper where they report a 50% yield Ullman methoxylation of ethylenedioxy analogue
of 6-bromopiperonal, i.e 7-Bromo-2,3-dihydro-1,4-benzodioxin-6-carboxaldehyde, http://www.sciencedirect.com/science/article/B6THS-453BS8F-J... . The Frenchmen have no experimental in their letter but they do refer to the
method in the paper http://www.erowid.org/archive/rhodium/chemistry/345-tmba.van... as the method they use for methoxylation, their 50% yield is rather low, but IMHO
worth it if you can avoid resorting to protecting group. I'd like to see the report you mention. 
[Edited on 22-6-2010 by Sandmeyer]
[Edited on 22-6-2010 by Sandmeyer]
|
|
|
gardenvariety
Harmless

Posts: 41
Registered: 19-1-2010
Member Is Offline
Mood: No Mood
|
|
I didn't see a single paper out to disprove it, but rather commentary in 3 separate papers that found negligible yields on those substrates. I'll try
to dig them up over the next few days, because it's been awhile.
All that aside, that still wouldn't require ac. anhydride. Still curious.
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Producing the desired material via 2,5-dimethoxy toluene, via bromination, might be possible. (If that toluene is available!)
Such a procedure is used industrially to produce 3,4,5-Trimethoxy benzaldehyde, from p-hydroxy-toluene .
Mono-Bromination of the aromatic ring....should proceed at the 4 position. Thereafter, followed by dibromination, of the toluel end of the
molecule.
Treatment of the resultant 2, 5-dimethoxy-4-bromo- BenzalBromide. With Potassium methoxide, then might proceed to eventually produce the 2,4,5-
trimethoxy-benzaldehyde.....
4-Bromination of the 2,5-dimethoxy benzyl- function....is well documented. This reaction has been used for the clandestine manufacture of DOB.
Others may have clearer insight into the possibility of using such a route. Asarone being pretty exotic; it may not be the best route for producing
its own aldehyde.
Also, I don't know where you are located, but Oxone in some form is probably available. It's a common pool/jacuzzi chemical.
It's sometimes sold retail under the name trade Oxone, but more often, it is sold under a more generic name.
There are many threads on the subject of Oxone, buried in the archives.
[Edited on 23-6-2010 by zed]
[Edited on 23-6-2010 by zed]
[Edited on 23-6-2010 by zed]
[Edited on 23-6-2010 by zed]
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
No need to complicate, a gentleman makes this compound from OTC chemicals, namely from vanillin via methylation to veratraldehyde, Baeyer-Villager
(peracetic i.e H2O2/AcOH), NaOH hydrolysis, metylation again to obtain 1,2,4-trimethoxybenzene, now, you use a sulfuric Ullmann variant of Duff
formylation (see sticky thread). The substrate should be activated enough for this formylation if it does not work try to formylate the phenol and
metylate as final step. All OTC...
|
|
|
Methansaeuretier
Harmless

Posts: 45
Registered: 24-5-2010
Location: Europe
Member Is Offline
Mood: strongly alkaline
|
|
Thank you all!
Quote: Originally posted by Sandmeyer  | | No need to complicate, a gentleman makes this compound from OTC chemicals, namely from vanillin via methylation to veratraldehyde, Baeyer-Villager
(peracetic i.e H2O2/AcOH), NaOH hydrolysis, metylation again to obtain 1,2,4-trimethoxybenzene, now, you use a sulfuric Ullmann variant of Duff
formylation (see sticky thread). The substrate should be activated enough for this formylation if it does not work try to formylate the phenol and
metylate as final step. All OTC... |
What's your your prefered OTC methylation agent?
To go from 4-Bromo to 4-Methoxy via sodium methoxide sounds much more attractive to me, as theres no need of using highly toxic methylating agents.
I'll have a look around if 2,5-Dimethoxytoluene is available for me... bromination, methoxylation and oxiation to the aldehyde sounds pretty easy.
Thank you zed.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
You could have said already in the beginning that all you want is the product and spared us the ode to asarone. Why bothering us with questions about
the oxidative cleavage of asarone if your problem is actually the preparation of 2,4,5-trimethoxybenzaldehyde by any means? BTW, are you aware that it
is commercially available?
Quote: Originally posted by Methansaeuretier  | To go from 4-Bromo to 4-Methoxy via sodium methoxide sounds much more attractive to me, as theres no need of using highly toxic methylating agents.
I'll have a look around if 2,5-Dimethoxytoluene is available for me... bromination, methoxylation and oxiation to the aldehyde sounds pretty easy.
Thank you zed. |
About 4-bromo-2,5-dimethoxytoluene and Ullmann: In comparison to the aldehyde or phenolic OH groups, the ortho-methoxy group is a very poor copper
chelator and thus Ullmann condensations with o-haloanisoles in most cases do not perform well, unless very efficient external ligands are used.
Exceptions are Ullmann condensations with tiolates and nucleophiles that themselves act as efficient Cu chelators.
About 6-bromoveratraldehyde and Ullmann: The aldehyde group is a moderately good copper chelator and thus the Ullmann condensations on such substrates
(o-bromobenzaldehydes) do indeed work and there are relatively many examples in the literature (best ortho chelators for Cu are the COOH and NO2
groups, but phenolic OH is nearly as good). However practically all these examples use nucleophiles with lower basicity (phenoxides, thiolates...).
The example given by Sandmeyer is practically the only one where a strongly basic nucleophile is used and this leads me to believe that the major
problem here is the competition with the Cannizzaro reaction. While the deprotonated vanillin derivatives are notoriously stable in regard to the
Cannizzaro reaction and the phenolic OH an excellent ortho-chelator for Cu, the same can not be said for the veratraldehydes and the aldehyde group.
In short, I think the Bayer-Villiger on veratraldehyde, O-methylation and Vilsmeier-Haack formylation as proposed by Sandmeyer is a more convenient
and reliable route. Mainly because all the steps are already described in the literature, while the use of the Ullmann's sulfuric Duff formylation is
a reasonably good and potentially working alternative to the Vilsmeier-Haack.
[Edited on 23/6/2010 by Nicodem]
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Quote: Originally posted by Methansaeuretier  |
What's your your prefered OTC methylation agent?
To go from 4-Bromo to 4-Methoxy via sodium methoxide sounds much more attractive to me, as theres no need of using highly toxic methylating agents.
I'll have a look around if 2,5-Dimethoxytoluene is available for me... bromination, methoxylation and oxiation to the aldehyde sounds pretty easy.
Thank you zed. |
Obviously you are not even capable of opening the thread I mention, in which Ullmann discuss the preparation and use of OTC methylating agent as well
as OTC formylation. Good luck with your endeavours, you'll need it...
| Quote: | All I want is the benzaldehyde, I'm not planning to make the amphetamine  forget
your dirty thoughts! forget
your dirty thoughts! |
Yeah, right. 
|
|
|
Methansaeuretier
Harmless

Posts: 45
Registered: 24-5-2010
Location: Europe
Member Is Offline
Mood: strongly alkaline
|
|
| Quote: | [quote=180718&tid=14038&author=Nicodem]You could have said already in the beginning that all you want is the product and spared us the ode to
asarone. Why bothering us with questions about the oxidative cleavage of asarone if your problem is actually the preparation of
2,4,5-trimethoxybenzaldehyde by any means? BTW, are you aware that it is commercially available?
|
The product isn't all I want.
And I like to make it, not to buy it. The journey is the reward.
And I'll try the oxidative cleavage of asarone in any case, because I have about 100g of ~90 % asarone which only needs to be destilled once more and
an ozone-generator is already ordered.
Anyhow the other way sounds also interesting, because I've never done something like that before.
BTW. 100g 2,4,5-Trimethoxybenzaldehyde from aldrich costs more than 140€. For 140 € I can buy at least 1100 ml of 45-80 % calamus oil and maybe
produce more than 500 grams in one day.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Now that I think of it, the modified Duff formylation of 1,2,4-trimethoxybenzene should proceed already in plain acetic acid (without the sulfuric
modification). At least this is the case with 1,3-dimethoxybenzene (UTFSE), which is only slightly more activated (it doesn't have the inductively
deactivating and resonance non-participating MeO group of 1,2,4-trimethoxybenzene - the one that later becomes meta to -CHO).
The retro-Henry condensation mentioned by Zed is nice idea to test. The required 1-(2,4,5-trimethoxyphenyl)-2-nitropropene can be prepared by nitrosation of alpha- or beta-asarone following by treatment with a base.
Edit: The references for the vanillin=>veratraldehyde=>1,2,4-trimethoxybenzene route are Helvetica Chimica Acta, 47 (1964) 1996-2017
and Synthetic Communications, 39 (2009) 3693-3709.
[Edited on 23/6/2010 by Nicodem]
|
|
|
| Pages:
1
2 |
|