Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Advanced Super Acid Chemistry
Can I suggest that
(CF3)3SbO , triflic anyhydride (FSO2)2O, and HOSO2CF3 will react to form the acid H(+) Sb(CF3)3(OSO2CF3)3(-).
This acid would be a useful superacid, much safer because it will not hydrolyze into toxic hydrogen fluoride gas. I would like to point out that I can
pour concentrated perchloric acid on my skin if I quickly wash it off because a protective layer of amine perchlorates (that has a low solubility)
form. If the danger of HF can be eliminated from a certain type of superacid, it might be possible to do the same. I'm not suggesting playing around
with the stuff, just that it could be less of a danger than HSbF6
Sb(CF3)3 has already been prepared.
"Bis(trifluoromethyl)cadmium complexes react with antimony trihalides in polar solvents to form Sb(CF3)3 in high yield." Dieter Naumann, Wieland Tyrra
and Ferdinand Leifeld
I am currently aware of H+B(CF3)4-, and carborane but these super-acids are expensive or not readily obtainable. H(+) Sb(CF3)3(OSO2CF3)3(-) could more
readily be prepared and useful in the lab.
I think that BF4- might react with SF4, although I think most chemists would think this is impossible. Although BF3 is a stronger Lewis acid, in this
case SF4 may act like the Lewis acid.
BF4- and 4 SF4 --> B(SF5)4-
B(CF3)4 (-) anion has already been prepared and found to hold its F- more strongly than even SbF6-. B(SF5)4- should be even better, possibly more
than carborane anions, allowing stabilization of new extreme cations not currently possible.
I would not agree that BF4- is inert, it likely is fairly reactive, but the equilibrium usually favors BF4-, thus it should be able to temporarily
donate a F- to SF4. Then the SF5- can stick back on to the boron. B(SF5)4- would likely be very non-coordinating. The parent compound SF6 is highly
inert.
Of course SClF5 is quite reactive and could be used to introduce the SF5 group to the Boron atom.
Have you considered B(CF3)4- ion? yes, it has been made, formed by B(CN)4- with ClF3
The corresponding acid H+B(CF3)4- is more acidic than HSbF6!
B(CF3)4- anion is known to hold on to its fluoride ion more than even SbF6- , that is to say that the parent compound B(CF3)3CF2 (I'm not sure if the
parent compound is real or hypothetical, but it was printed in some literature) is a stronger F- abductor than even SbF5. I believe it may have been
your group that created N5+B(CF3)5- by precipitating out CsSbF6, in an attempt to create a more stabilized pentazolium salt.
The thought occurred to me, if B(CF3)4- is better than SbF6-, than would not Sb(CF3)6- be even less coordinating? Possibly this could be done by using
(CH3)4N+, Sb(CN)6- and reacting with ClF3. As you already well know (CH3)4N+ is not attacked by ClF3. Then the tetramethyl ammonium cation would be
exchanged for a hydrogen cation, leaving HSb(CF3)6, perhaps even more acidic than carborane acids.
And since Au2F10 is a stronger F- abductor (on wikipedia) than SbF5, perhaps Au(CF3)5(CF2), if it could be made, would be an extremely strong fluoride
ion abductor, possibly enabling the stabilization of new extreme cations. Au(CF3)5(CF2) may be more likely to exist than Sb(CF3)5(CF2) because since
AuF5 is electron deficient enough to dimerize, the gold could possibly share electrons with the carbon, which is only otherwise bound to the two
fluorine atoms.
As a final note, I am certain you already know, a new crystal allotrope of nitrogen was created under extreme pressure. The interesting thing was
that it maintained its solid state when brought back to atmospheric pressure, so long as the temperature was first reduced while under pressure and
maintained at liquid nitrogen temperatures, not enough to freeze ordinary liquid nitrogen. This may have some bearing on your team's attempt to
synthesize N5+N5-.
Hopefully some of this will give someone some ideas-
Perhaps B(SF5)4- anion is possible. SF6 does not even react with molten elemental sodium, very non-coordinating. Also if you know anyone interested in
nitrogen chemistry:
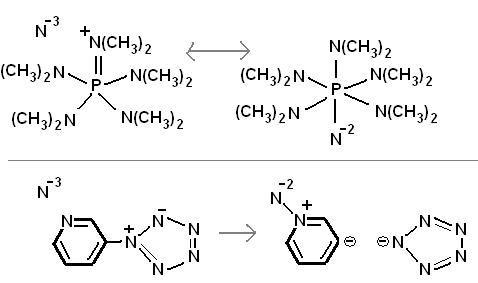
superbase
in the picture you can see a theoretic superbase more powerful than Li3N. Li3N is not completely ionic. Did you know metallic sodium will not burn in
nitrogen so Na3N has not been prepared in any significant quantities before? They are trying to make ionic nitrogen allotrope. They (ex. Karl Christe)
already made N5+, now they are trying to make N5-. They calculate that the two should form a salt. Unfortunately N5+ oxidizes azide.
[Edited on 16-6-2010 by Anders Hoveland]
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
This is an interesting read and its about very special compounds! It would be really surprising if N5N5 could be prepared, another allotrope of
nitrogen!
Unfortunately this kind of chemistry is well beyond what a home chemist can do. I really would love to experiment with that kind of stuff. I also have
heard before of the person 'Christe'. Apparently that guy is very much into preparing cutting edge compounds.
I recently read something about another cutting edge compound, the so-called dinitramide ion, N3O4(-), having structure N(NO2)2(-). Apparently this
ion is quite stable and inert (like perchlorate) but its preparation is very difficult (another interesting example of a very stable but extremely
difficult to prepare ion is perbromate). Do you have information on this dinitramide ion? It really looks like something which eventually could be
done by a home chemist, but of course I might be totally wrong.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
I am so glad you found my post interesting. I love to share my ideas, but it is so hard to find people that can understand it all and have an
interest.
Dinitramide is not that difficult to make. Sulfamic acid (5%) is the ingredient in some toilet bowl cleaners, see your drug store. Sulfamic acid can
be nitrated, forming (NO2)2NSO3H, which will decompose after only a few minutes, so you must quickly neutralize with NaOH to form NaN3O4. Sulfamic
acid can also be made from SO3 and anyhydrous NH3, the reaction is rather violent. I forget the the rest, about fractional crystallization, how to
make NH4N3O4 from the potassium salt, but I believe it used ethyl alcohol. NH4Cl and NaClO4 can be used to make NH4ClO4, which has a surprisingly low
solubility for an ammonium salt, less than NaCl.
NH4N3O4 while slightly more energetic than NH4ClO4, is significantly more sensitive. While it can be used as rocket fuel oxidizer, there is a
significant chance of explosion.
Perhaps explore hydroxylamine nitroformate (my idea) which should be more stable and energetic.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Interesting to read that dinatramide can be made fairly easily. This is something I certainly will try. I do have sulfamic acid and I have conc. HNO3
(65% and also some 90+%). Can sulfamic acid easily be nitrated? I never read about that and never tried that. In fact, I considered sulfamic acid a
fairly tame chemical which is not that interesting. Now I see new interesting options for this chemical.
The things you find interesting are the things which I also find very interesting. I try to find/make peculiar (usually covalent) compounds and
investigate their properties. For this reason my favorite experimenting area is in the upper right corner of the periodic table and combining the
elements in that part of the periodic table in all kinds of peculiar compounds.
Just as an example, an interesting compound I made (but not isolated) is the SO2 adduct of iodide ion. This is not well known at all, but just for fun
you should add a little acidified sulfite (e.g. Na2SO3 dissolved in some dilute H2SO4) to a solution of KI in water. No reaction is expected, but the
solution turns yellow and a complex is formed with formula [I.nSO2](-). A solid form can be prepared by dissolving KI in liquid SO2 and then
evaporating the excess SO2. A yellow solid remains. Unfortunately as a hobby chemist I don't have the equipment to make liquid SO2, I only read about
this.
Maybe the following pages from my website are interesting for you:
http://woelen.homescience.net/science/chem/riddles/
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
I have a different take on your "compound". I think that HI is formed, gets oxidized by SO2, forming iodine and sulfur. The sulfur reacts with the
sulfite to form thiosulfate, which then gets oxidized by iodine to the tetrathionate S4O6 -2.
SO2 can be both an oxidizer and a reducing agent. For example, I think it might react with H2S at room temperature. Alternatively it can reduce
nitrous acid to hydroxylamine, through some interesting intermediate arrangements. An idea I had was to use CH3NO with bisulfite to make CH3NHOH. I
am almost certain the CH3NO2 would NOT be reduced, however.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I do not agree with your explation of the yellow acidic sulfite/iodide reaction.
First: your explanation does not explain the yellow color. In your explanation, iodine only exists in a transient state, but the observation is that
the yellow color persists.
Second: The reaction only occurs at low pH when there is free SO2. It also occurs in liquid SO2 where there is no sulfite at all. So, formation of
thiosulfate from sulphur and sulfite is not possible at all in such media. At low pH, thiosulfate is unstable and the corresponding acid decomposes to
SO2, S and H2O.
Third: No turbidity can be observed at all. if any sulphur would be formed, then the liquid would become opaque, at least opalescent.
I know that SO2 can act as an oxidizer and for me, this also was my first thought when I observed the yellow color on addition of iodide to sulphur
dioxide. But doing more and more experiments with this, I simply came to the conclusion that this cannot be the explanation of the observations. With
the help of another guy (in Pakistan) we finally settled the issue. He found an old 1960-paper about a totally forgotten reaction.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
When you acidify thiosulfate, it turns yellow, but opaque. I would have thought that HI reacts with SO2. I can't imagine it not going opaque when SO2,
iodide, and acid is present. But I would not be surprised if (SO2I) -2 formed. It has little interest for me, as it cannot be used to make anything
extreme or useful.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
SO2, iodide and acid, when brought together give no opacity, the liquid just turns bright yellow, but remains clear.
|
|
|
|