Synthesize Me Captain
Harmless

Posts: 4
Registered: 24-5-2010
Member Is Offline
Mood: No Mood
|
|
Isopropyl Alcohol and Alkaloid Reactivity in Water
To start, I will say I've taken a general chemistry course in college and have found it very interesting. I have done some experimentation but I have
not yet taken an organic chemistry course, so please forgive me and feel free to correct me if my information is wrong.
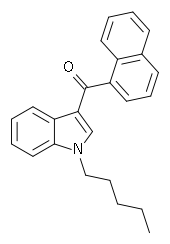
My experience and question is with the compound Naphthalen-1-yl-(1-pentylindol-3-yl)methanone, commonly known as JWH-018 (C24H23NO). It is a
hydrophobic, synthetic cannabinoid, and it is perfectly legal. But I am not here to discuss the topic of drugs or to discover how to synthesize my
own- I'd like to examine interactions between it and other substances.
I had vaporized approximately 30 mg of the substance in a 100 ml volumetric flask, but was certain I still had some that had not vaporized.
Remembering that it was soluble in alcohol, I used approximately 12 ml of isopropyl alcohol to absorb it. I then wondered what would happen if I
vaporized the alcohol and then allowed it to condense as it cooled to maximize solubility. I used a home made ring stand and bunsen burner to achieve
this.
Now at this point the solution was completely clear. I decided to dilute the solution for later use, but had no distilled/deionized water, so I had to
settle for tap water. As soon as I added the water, a milky white precipitate formed and the entire solution soon became cloudy and uniformly white.
The original solution was still slightly warm, but I doubt it had any impact by this point.
I am very intrigued by this, as I thought I understood isopropyl alcohol to not react with water, and not as the solvent for JWH-018. And then I
considered the fluoride and chlorine present in tap water. But still, I cannot understand why it would react. There was also an audible noise when the
two solutions were mixed, and the coloration was very noticeable with only 1 drop of water.
Could I have synthesized a new compound, and if so, what could it be? I tested further by normal mixing of water/isopropyl and another alkaloid,
dimenhydrinate (dramamine). There was no change in appearance in the solution. Can anyone please outline just what may have happened in this
experiment?
(As one more note, I tested this solution in a small quantity to avoid toxicity from the isopropyl alcohol. The effects were noticeable sooner and
with a smaller dose, but the experience seemed shorter in duration.)
|
|
|
Element
Harmless

Posts: 3
Registered: 5-4-2010
Member Is Offline
Mood: No Mood
|
|
I'm really not yet an expert, but it seems that it is the ketone that is problematic... JWH-018 has a pretty obvious one, while dramamine's is
stabilized in a (aromatic? All those groups confuse me, sorry  ) ring. ) ring.
Ketones react with water to form alcohol, that's probably why... but some people here have a lot more knowledge of organic chemistry 
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Your first step should be to mix your tap water with IpOH and see if you still get a precipitate because my first guess would be that your just
pushing a mineral, more then likely a Ca salt, out of solution with the IpOH.
"The effects were noticeable sooner and with a smaller dose, but the experience seemed shorter in duration."
You where inhaling IpOH. Much of what you experinced was more then likely Alcohol intoxication.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Are you sure the white material is not just the compound crashing out? It would depend on the solubility of it in water.
| Quote: |
I'm really not yet an expert...
...Ketones react with water to form alcohol, that's probably why... |
I've never heard so much bullshit in all my life. You've got a long way to go before you're anything comparible to an expert. The closest truthful
thing would be that it forms a *hydrate*, not an alcohol.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: |
| Quote: |
I'm really not yet an expert...
...Ketones react with water to form alcohol, that's probably why... |
I've never heard so much bullshit in all my life. |
That's a bit harsh DJF90 - it's only his third post and he's admitted his lack of expertise already. . .
|
|
|
Synthesize Me Captain
Harmless

Posts: 4
Registered: 24-5-2010
Member Is Offline
Mood: No Mood
|
|
Sorry about the delay- I'm a bit of an insomniac but still had to sleep some.
I have already mixed the tap water with the isopropyl alcohol by itself. There are no indications of any precipitate forming. Also, the consumption
was strictly oral, with no inhalation of fumes (and if that were the case, it was a weak concentration).
| Quote: | | Are you sure the white material is not just the compound crashing out? It would depend on the solubility of it in water. |
I have thought about this too, but my ignorance holds me back. It is insoluble in water, so it would make sense that 100 ml of water to such a small
amount would force it out of solution (I think). Yet, there is no way there is more than 10 mg of compound remaining, and one drop of water
immediately caused a change- I wouldn't think it would fall out of solution until more was added (again, not sure of that). There is no sediment; the
solution is uniformly white and looks just like milk. So I still think there is a reaction taking place.
I won't go into details, but I have noticed a similar effect with isopropyl alcohol and water with an amphetamine solution. I just can't figure out
the connection.
[Edited on 24-5-2010 by Synthesize Me Captain]
[Edited on 24-5-2010 by Synthesize Me Captain]
|
|
|
azo
Hazard to Others
  
Posts: 163
Registered: 12-2-2008
Member Is Offline
Mood: No Mood
|
|
what makes you think there is a reaction between jwh-018 and water as dJF90 said ketones don't react with water to form alcohols there is a posability
of calcium or magnesium ions reacting with the said functional group to prove this you could add a small amount of chelating agent like edta or sodium
try polyphosphate to the water first before adding to alcohol which will seqenture hardness ions and stop them interfeiring with the jwh-018
As for the milky look water and lower aliphatic alcohols tend to turn milky.
?what has this got to do with amphetamine ? is there a message here .
i am sure there are other members with a lot more experience than me that could help you.
regards azo
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Alcohols in general are not reactive towards anything that doesn't react with the H-O group, compounds that do so also react with water. A search of
the literature (as such) shows instances of JWH-018 used in ethanol-water solution, suggesting no reaction. The molecular structure is not one I'd
expect to readily react with ROH compounds including good old HOH.
At this point I'd say you need to repeat using distilled or deionised water, and check the purity of your isopropanol. Note that low concentrations
of water insoluble substances in water-miscible solvents will frequently give dispersions of the substance whem mixed with water - milky in appearance
and often slow to clear as the small droplets or crystals do not sink or float quickly. Adding more of the solvent clears the milkyness as it
increases the solvent power of the mixture.
Ketones and aldehydes react with water to give hydrates, which are geminal diols and thus alcohols, and with alcohols to give hemiketals and
hemiacetals, then ketals and acetals (I guess that the term 'ketal' has falling out of usage. Acid catalysts are needed, and for most aldehydes and
ketones the reaction is very unfavorable
R'R"C=O + H2O <<<=> R'R"C(OH)2 and R'R"C=O + ROH <<<=> R'R"C(OH)(OR) ; + ROH <=> R'R"C(OR)2 + H2O
The acetal or ketal formation can be forced by removing the water formed.
Only a small number of carbonyl compounds have reasonably stable hydrates and hemiacetals. These include strained ring ketones such as
cyclopropanone, or have strongly electron withdrawing groups alpha to the carbonyl - chloral, gloxyal, 1,2,3-indatrione (ninhydrin), or form stable 5
or 6 member rings - 5-hydroxypentanal for example and many sugars do this.
The existence of hydrates and hemiacetals for most aldehydes and ketones can be demonstrated through oxygen exchange with the hydroy compound, but
they can not be isolated as such.
| Quote: | | and it is perfectly legal. |
Apparently it is not legal in a number of countries, and some States of the USA. YMMV, but I'd not bank on the legal status of this compound and its
kin, particularly in a certain country that at times has banned both ethanol and trans-fatty acids "for your own good"
[Edited on 25-5-2010 by not_important]
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
The material is designated as being "hydrophobic". This suggests that it is normally a liquid. Were it a solid, it would be stated that it is
"insoluble" in water.
So, you mixed it with a polar solvent (2-propanol) and a clear solution formed.
Then, you made the solution much more polar, by adding water to it.
Surprise, surprise, the oil crashed out of solution. Technically, I suppose it formed an emulsion. In the most rigid sense, it is not actually a
precipitate. As the term precipitate, is generally used, to refer to solids.
So, remove the water, which will render the solvent less polar, and the hydrophobic material will go back into solution.
[Edited on 26-5-2010 by zed]
[Edited on 26-5-2010 by zed]
|
|
|
Rich_Insane
Hazard to Others
  
Posts: 371
Registered: 24-4-2009
Location: Portland, Oregon
Member Is Offline
Mood: alive
|
|
Nope. JWH-018 is a solid. It is hydrophobic, which I think he means as a synonym for insoluble in water. I highly doubt there's a reaction. When I get
hot tap water from the faucet, it appears very milky at first. Try your experiment with distilled water. Make a control, just try dissolving the stuff
in pure alcohol. If it is indeed hydrophobic, it should have increasing solubility with longer chain alcohols. Cannabinoids are very, very variable in
experience. You're going to want to get a really solid control from multiple subjects and average out the intensities if you want to test that way.
|
|
|
Synthesize Me Captain
Harmless

Posts: 4
Registered: 24-5-2010
Member Is Offline
Mood: No Mood
|
|
I find all of this very interesting and helpful- thanks!
|
|
|
Synthesize Me Captain
Harmless

Posts: 4
Registered: 24-5-2010
Member Is Offline
Mood: No Mood
|
|
I haven't tested further but have come across some disturbing effects. These could be due to one of two things. Each time I have sampled more the
effects have become stronger, even with smaller amounts. I am taking bupropion (a norepinephrine and dopamine reuptake inhibitor) and only started
about a week ago. It could be that the solution itself is unchanged, but my medication is greatly altering the affects.
When JWH-018 acts on the body it can cause tachycardia and other reactions from the sympathetic nervous system. I now believe it also signals the
release of norepinephrine, because a small amount induced a panic attack. I thought perhaps it was just a more concentrated sample, until I couldn't
sleep following a dose and took 1 dramamine pill to make me drowsy (50 mg dimenhydrinate). Within about 45 minutes I became completely disoriented and
had another panic attack with effects similar to JWH-018- or maybe it is more accurate to say isopropyl alcohol. But these are such small amounts that
I have to say bupropion almost seems to act as a monoamine oxidase inhibitor.
Either way I am staying away from these substances for a long while as the solution may be toxic. Maybe it's just my NDRI, but feeling on the verge of
dying is not something I wish to repeat- if you're going to experiment with psychoactive chemicals, you better make damn sure you know what you have
on your hands and use all due caution, especially if it is a research chemical with little known about its complete mechanism of action.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Panic attacks aren't unusual with regular THC. Many people reach a point where they simply refuse to partake. "It makes me Paranoid!"
Some folks have similar problems with coffee.
Good reason to take a break. Some part of you relishes getting stoned, while other parts.... clearly have decided that you've had enough.
JWH-018 is cheap enough. This might be a good time to flush it.
|
|
|