| Pages:
1
2 |
agorot
Hazard to Self
 
Posts: 73
Registered: 25-1-2010
Location: too fed-o-phobic to say :D
Member Is Offline
Mood: like an activated complex
|
|
Ammonia by the haber process--tabletop chemistry
Although ammonia is readily attainable from hardware stores with ammonium sulfate and a strong base, I was curious when I read this on wikipedia:
| Quote: | | The enormous technical problems associated with the process were first solved by German chemist Fritz Haber (with the invaluable help of Robert Le
Rossignol, who developed and built the necessary high-pressure devices). They first demonstrated their success in the summer of 1909, producing
ammonia from air drop by drop, at the rate of about a cup every two hours. The process was purchased by the German chemical company BASF, which
assigned Carl Bosch the difficult task of scaling up Haber's tabletop machine to industrial-level production. |
I've searched online and can't seem to find a schematic for this "machine." It must have been pretty intense though.
I found this book that explores the topic, but I have no idea if it actually goes into the process that haber origionally used instead of just
exploring the history.
"The Alchemy of Air: A Jewish Genius, a Doomed Tycoon, and the Scientific Discovery That Fed the World but Fueled the Rise of Hitler"
The following are also good websites
http://haberchemistry.tripod.com/
http://en.wikipedia.org/wiki/Haber_process (note it seems the original hydrogen source was electrolysis of water, something easy to do)
Now everyone, lets not just shoot this down immediately. If your first inclination is to warn me, "some idiot kid that doesn't know what he must be
doing", about safety, then please just don't respond. We're just exploring the chemistry. I'm aware of the safety risks/dangers of the chemicals,
pressures, and temperatures involved. Be constructive.
[Edited on 11-5-2010 by agorot]
[Edited on 11-5-2010 by agorot]
|
|
|
roamingnome
Hazard to Others
  
Posts: 363
Registered: 9-9-2006
Member Is Offline
Mood: No Mood
|
|

I believe it is quite possible. You may have seen pictures of the original apparatus.
The French techniques have worked with higher pressures up to 10,000 psi in small diameter tubes which give a larger single pass yield of ammonia.
Technology these days is left for the large corporation that will safely deliver products to the consumer.
There is alot of details to consider of course
|
|
|
agorot
Hazard to Self
 
Posts: 73
Registered: 25-1-2010
Location: too fed-o-phobic to say :D
Member Is Offline
Mood: like an activated complex
|
|
I don't suppose anyone is aware of any schematics available online?
|
|
|
grndpndr
National Hazard
   
Posts: 508
Registered: 9-7-2006
Member Is Offline
Mood: No Mood
|
|
Not here to criticize! are you considering an attempt to duplicate
the method if not the exact apparatus used? Looks fascinating but i would expect the need of a very good milling machine,shaper and lathe combined
with the talent of a tool and die maker as well as heat treat equipment.Quite an expensive /difficult undertaking and possibly quite dangerous given
the working pressure.I understand the appeal however.Wish i could provide concrete assitance but far beyond me.good luck in any endeavor agrogrot!
|
|
|
agorot
Hazard to Self
 
Posts: 73
Registered: 25-1-2010
Location: too fed-o-phobic to say :D
Member Is Offline
Mood: like an activated complex
|
|
yeah, you may be right. I do want to find out first though if it is completely out of the question. I'd be willing to throw down a couple hundred
bucks, but that may not cover it. it would be awesome to be able to make something like this, especially because it is on such a small scale
|
|
|
densest
Hazard to Others
  
Posts: 359
Registered: 1-10-2005
Location: in the lehr
Member Is Offline
Mood: slowly warming to strain point
|
|
The various web sources say 400-450C and 200bar = 3000psi ... I've done a little plumbing for 150bar O2 at 20C and that made me nervous enough!
The famous tabletop experiment had to have been fed from external high pressure and heated somehow. The process is exothermic - I don't think it's
self-sustaining. There also must have been insulation covering the hot part of the process as well.
A hobbyist version using tanks of H2 and N2 from the local welding shop would take care of the pressure. 100bar would be enough to show it working.
Heating the apparatus with an electric mantle would work. Cooling the output below 110C under pressure would liquefy the NH3 and allow separation, but
much more cooling would be required to keep it liquid under atmospheric pressure so it could be seen.
2000psi plumbing parts can be purchased for only mildly extortionate prices. Depending on them at 400C is something else again. Keep the enclosed
volume as small as feasible - 1-2mm ID tubing, for instance. The reaction (catalyst) chamber might be a piece of 2000psi iron pipe?
|
|
|
agorot
Hazard to Self
 
Posts: 73
Registered: 25-1-2010
Location: too fed-o-phobic to say :D
Member Is Offline
Mood: like an activated complex
|
|
Quote: Originally posted by densest  | The various web sources say 400-450C and 200bar = 3000psi ... I've done a little plumbing for 150bar O2 at 20C and that made me nervous enough!
The famous tabletop experiment had to have been fed from external high pressure and heated somehow. The process is exothermic - I don't think it's
self-sustaining. There also must have been insulation covering the hot part of the process as well.
A hobbyist version using tanks of H2 and N2 from the local welding shop would take care of the pressure. 100bar would be enough to show it working.
Heating the apparatus with an electric mantle would work. Cooling the output below 110C under pressure would liquefy the NH3 and allow separation, but
much more cooling would be required to keep it liquid under atmospheric pressure so it could be seen.
2000psi plumbing parts can be purchased for only mildly extortionate prices. Depending on them at 400C is something else again. Keep the enclosed
volume as small as feasible - 1-2mm ID tubing, for instance. The reaction (catalyst) chamber might be a piece of 2000psi iron pipe?
|
200 bar is what is used on the industrial scale, but if you read on, haber's machine produced only about a cup of NH3 per hour, which leads me to
think that in his design, he used high pressure, but not quite that high, and not quite that hot either. I'm not sure though, that's why I'm trying to
find out more about his original design.
You're right that the rxn is not self sustaining. It does need to be heated, unless haber origionally did it at room temp? Since it is exothermic,
LeChatlier tells us that actually lower temperatures are more efficient, but that does not mean that they will produce more NH3. Perhaps
Haber was going for efficiency in his earliest design so he did it only at room temp.
I've read that the catalyst is actually magnetite, so iron ion...
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
As well as high pressure, it would, ideally, require a transition-metal catalyst which absorbs large volumes of N2 and H2 at such pressures, thereby
becoming the reaction substrate. Pd would be ideal for this, although at such high pressures, much cheaper Ni can be made to substitute for it, and
probably others such as Fe.
[Edited on 12-5-10 by JohnWW]
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Thank you for mentioning
budget relatively early in the discussion. It's critical for any sane discussion of small-scale engineering. I wish, largely in vain as of now, that
this were the norm. I really doubt that $200 will be enough. It's enough to cover some personal protection gear, though, so it's enough to get
started. At the very least you're going to want a respirator with an ammonia-specific cartridge.
The bulk of the cost of such a unit is going to be the pumps and compressors involved. The N2/H2 recycling loop is one example. You've got to provide
some constant positive pressure differential across that pump, even if your flow rate isn't that high. The bigger practical problem is a pump that
won't explode. You've got three relevant pressures here: one each before and after the catalyst bed and the third is atmospheric. Even if you only
need (say) 1 bar difference across the catalyst bed, you've got the full 100-200 bar difference from the innards of the pump to the outside. A
bottled-gas compressor, mostly easily available for scuba tanks, can do this for inert gas, but they aren't cheap. (Even then, they'd likely need new,
chemically-compatible seals.)
An alternate way of getting an adequate pump would be to stick an ordinary pump inside a pressure vessel. It's all about ΔP, so if you
eliminate that, a cheap pump might work. The only problem is that you need a pressure vessel, one in two parts with a pair of flanges that you can
disassemble. Depending on what industry there is where you are, this might be available as scrap or surplus. Unless you get very lucky, you'll likely
need to weld in this scenario.
Other costs are special materials for chemical compatibility. You've got hot hydrogen, which embrittles many common steels. Look up "hydrogen
embrittlement" for more on the subject. It's a pretty good way to get a sudden explosion with flash-off of a lot of hot ammonia; not fun. Please do
check that you've got compatibility at operating temperature, not just at room temperature. You might be able to get away with a material with
marginal service life, but if you're willing to tolerate disassembly and inspection relatively frequently.
Personally, I'd recommend building and testing the apparatus in components. Take the separator that extracts NH3 from the exhaust from the catalyst
bed. A version of this that actually worked, even if fed with a test gas mixture (bottled H2, N2, NH3, for example), would constitute progress in my
book.
|
|
|
roamingnome
Hazard to Others
  
Posts: 363
Registered: 9-9-2006
Member Is Offline
Mood: No Mood
|
|
I've aquired a number of papers about ammonia
but this seems like it will have some real parameters to start chewing on
In reality ammonia synthesis is changing to the future.....
proton conducting ceramics coated with palladium produce ammonia at normal pressure..ill try to dig up the european patent its quite interesting and
doeable..
Attachment: hn3jpdf.pdf (256kB)
This file has been downloaded 1176 times
[Edited on 13-5-2010 by roamingnome]
Attachment: EP0972855B1.pdf (82kB)
This file has been downloaded 1053 times
i hope these "chip" style generators become more common place. Im always a long way off, but i did get me some cerium oxide, but the company just
kept draggin its butt on the
Yb nitrate. anyway good luck
[Edited on 13-5-2010 by roamingnome]
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
I wonder how high the pressure can be driven with a refrigerator-compressor: If it's opened and the motor is substituted/hacked/better-cooled/etc. :
Might it reach 50 bar ? How far above that ?
Of cource the entire experiment would have to be safely contained: Get some bricks and mortar, maybe 2 webcams ...
Piping can hold _lots_ of pressure as long as the diameter is small: Glass-tubes of 1 mm wall-strength and inner diameter of 2 or 3 mm will go up to
500 bar or above ... ; ideally quartz-glass would be used, which holds the pressure at high-enough temperature ...
==> The volume of the reaction would be distributed over lenghts of relatively thin tubing, laying side-by-side in the furnace ...
==> Of course: When a piece of the tubing blows it will take the rest with it ... !!! So: Bricks and mortar to contain it, and noone in the room
since such a setup will act as a canon for some of the pieces, throwing them violently !!
The pressure-resistivity of tubing depends on the disruption-tension of the material:
==> The same wall-strenght will hold the same forces ...; with smaller-diameter tubing these forces are smaller, since the pressure then acts onto
smaller surfaces ...
That's why a 5mm-tube will hold several times the pressure of a 1-cm-tube ..., at same wall-strength and material ...
==> Smaller plumbing will be aduequate for surprisingly high pressure ..., but safety first, as mentioned above ...
[Edited on 13-5-2010 by chief]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Well, let's look at the record
| Quote: |
This goal was realized by Fritz Haber in 1908[4, 5] when he was able to
produce ammonia in a substantial yield from nitrogen and hydrogen by the use of an
osmium catalyst at a pressure of about 175 bar and at 550 °C. Haber’s apparatus
produced about 90 grams of ammonia per hour with about 98 grams of the osmium
catalyst[1, 3, 6, 7]. Due to the cost of osmium, research into more economically
feasible catalysts was conducted. Then Alwin Mittasch of BASF in 1910 found that
adding alumina, Al2O3, and potash, K2O, to iron was a suitable catalyst for ammonia
synthesis.
At this point, there was a sizable gap between Fritz Haber’s apparatus and a
full-sized plant that would be able to mass-produce ammonia. The largest part of this
problem was the high pressure of reactant gasses that was needed to produce
ammonia. The technology to perform this high pressure reaction was developed by
Carl Bosch of BASF in 1913 who oversaw the first factory dedicated to ammonia
synthesis in Oppau, Germany[1, 8, 9]. This factory was able to produce 30 tons of
ammonia per day with 300 Kg of the doubly promoted iron catalyst with a total
pressure of 200 atmospheres of the nitrogen and hydrogen reactant gasses.
[1] Jennings, J.R., Catalytic Ammonia Synthesis Fundamentals and Practice.
[3] Topham, S.A., Catalysis, Science and Technology, ed. M.B. John R. Anderson. Vol. 7. 1985, Berlin: Springer-Verlag.
[4] Crookes, W., Report of the 68th meeting of the British Association for the Advancement of Science, Bristol, 1898. 1898, London: John Murray.
[5] Goran, M., The Story of Fritz Haber. 1967, Oklahoma: University of Oaklahoma Press.
[6] Haber, F., Z. Elek., 1910. 16: p. 244.
[7] Haber, F. and R.L. Rossignol, Z. Elek., 1913. 19: p. 53.
[8] Mittasch, A., Geschichte der Ammoniaksynthese. 1951, Weinheim: Verlag Chemie.
[9] Partington, J.R. and L.H. Parker, The Nitrogen Industry. 1922, London: Constable.
|
From the paper here
Model Catalysis of Ammonia Synthesis ad Iron-Water Interfaces - A Sum Frequency Generation
Vibrational Spectroscopic Study of Solid-Gas Interfaces and Anion Photoelectron Spectroscopic
Study of Selected Anion clusters
Ferguson, Michael James
12-15-2005
http://www.escholarship.org/uc/item/80v739hb
To me it sounds as if it should be the Haber-Mittasch-Bosch process.
Until recently iron-based catalysts were the only ones of interest, various such as using Rb or Cs in place of potassium, or adding some platinum,
were the sort of thing that showed up. Recently a ruthenium-based catalyst has entered the field, operating at lower pressures and temperatures. It
often is used in a multibed reactor with the first bed being conventional iron catalyst, which does well when the NH3 concentration is low. The Fe
catalyst is also much cheaper than the Ru one, so any poisons (halogens, S, As, P) that get into the feed streams are going to trash it first.
The reaction is exothermic, reactor designs use various methods of handling the heat. The designs are such that the temperature rises from ~400 C at
the injection point to ~500 C at the next cooling stage. In a very small reactor this would be less of a problem due to the decreased ratio of volume
to surface.
There have been recent developments on both the traditional Haber H2-N2 method, and several electrochemical method, some of which use N2 and steam as
their feedstocks. High temperature electrolysis can yield high purity H2 at lower energy costs than conventional electrolysis, in part by using the
heat created by the N2-H2 reaction in steam generation.
A paper related to the European patent roamingnome supplied
http://dx.doi.org/10.1006/jcat.2000.2877
a variant of that
http://dx.doi.org/10.1016/j.ssi.2005.07.018
at 50 bar using convention iron catalyst plus proton conductor
http://pubs.acs.org/doi/abs/10.1021/jp002236v
Some non-technical overview papers on solid state NH3 synth methods
Attachment: HowardUniv1.pdf (747kB)
This file has been downloaded 1076 times
Attachment: SSAS_Oct2007_Final.pdf (210kB)
This file has been downloaded 887 times
A research paper on a plant using ruthenium catalyst at ~100 bar
Attachment: nh3syn97.pdf (168kB)
This file has been downloaded 2414 times
United States Patent Application 20090202417 is for a nanoparticle form of the iron catalyst, claimed to work as low as 10 bar.
And an oddball paper, I sense a lot of handwaving and buzzwording, using a Mn-Zn-Fe ferrite at STP in a microreactor with a magnetic field. This may
be closer to running you automobile on water, than to anything useful, but hey - it's <s>Mad Science</s> Science Madness, you can try it
yourself. :-)
Attachment: Electromagnetic - Microreactor Design for Ammonia Synthesis.pdf (803kB)
This file has been downloaded 1218 times
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
not_important, it appears we've read many of the same publications about ammonia synthesis. The solid state electrochemical ammonia synthesis based on
steam and N2 is the one that interests me most, but I don't think it has been demonstrated even on a pilot plant scale yet. I keep checking every 6
months or so for news.
I think the capability to use stranded renewable energy is alluring but it also raises more questions. How quickly can SSAS respond to changing
availability of electrical power? What is the energy consumption including process heat and not just the electrochemical needs? How does thermal
cycling from intermittent use dictated by intermittent power affect equipment lifetimes?
Another intriguing aspect of SSAS is that it appears amenable to scaling down much more than conventional ammonia synthesis. A future opportunity for
opportunistic generation of NH3 at the very farm where the fertilizer is needed?
PGP Key and corresponding e-mail address
|
|
|
densest
Hazard to Others
  
Posts: 359
Registered: 1-10-2005
Location: in the lehr
Member Is Offline
Mood: slowly warming to strain point
|
|
The "Electromagnetic - Microreactor" paper leaves out one little tiny little detail - the excitation frequency. Also, the parameters for the coils
just don't make sense. Assuming the coils are 10cm in diameter and 2 cm thick in both dimensions and DC excitation, the coils are not physically
realizable. They show either 2 14 ohm in parallel or 2 3.5 ohm coils in series. In either case, there is no wire (except superconducting... ) which would have a low enough resistance and would fit (by a factor of 5 to 25 at
least). AC power would just make things even more improbable. ) which would have a low enough resistance and would fit (by a factor of 5 to 25 at
least). AC power would just make things even more improbable.
The aluminum reactor body would have some interesting eddy currents to waste power if the excitation is AC.
The nanoparticle ferrite just might do something - I dunno.
Anybody want a magnet for your automobile fuel line?
Going back to the porous ceramics...
The EU patent doesn't specify thickness of the ceramic or its conductivity at the reaction temperature. The discussion implies a very high resistance
since the current is very close to that required to perform the reaction alone. They don't report a voltage which would be very interesting - the
power into the ceramic just might heat it enough to keep it hot. One would like to see a blank (N2 or H2 only) run. A thin platinum film (perhaps
interacting with the ceramic in some unspecified way) might have substantial catalytic activity at 400-500C. They say that the rate is completely
current dependent, though, but one can conceive a way that a heated catalyst constantly swept with reactants might work without any electrochemical
mechanisms.
Just by inspection, it doesn't look like anything less porous than a zeolite would have enough surface area to support even the low reaction rate. I
haven't calculated it. One might expect a reactor with N2 & H2 on one side under a couple of bars pressure and the product being vacuumed out the
other side to make more sense.
Has anyone gotten the "Ionics" paper - reference 12 - to see how the plates were prepared?
10-8 mol/sec/cm2 is 0.6 g/hour/m2. That implies a lot of plates for even a demo version.
Looks like typical patent obfuscation.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
| Quote: | | They first demonstrated their success in the summer of 1909, producing ammonia from air drop by drop, at the rate of about a cup every two hours.
|
Did this happen to feature osmium?
Or was it uranium?
I'm confident that relevant references to the journals including the contemporary work with le Rossignol will be found in Mellor, and many BASF patent
numbers in Thorpe, and the patents will be found at espacenet.
I bet not a lot of people have made one.
EDIT: Wow, the French wikipedia is a lot better than the English one in this case. They make the one-paragraph history especially look like a joke. Do
they have some form of IQ test to post on the internet?
[Edited on 13-5-2010 by S.C. Wack]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
The apparatus of Haber-Le Rossignol is below. Described in Fritz Haber by Margit Szöllösi-Janze, this is the device Boesch came to
familiarize himself with in 1909 in Karlsruhe. There was a defective seal, so Boesch didn't see it in action then.
The reaction can be carried out at regular atmospheric pressure with basic chemistry set ups, but then the yield of ammonia will be significantly
less. Haber and others have studied the effect of temperature and pressure on the reaction equilibrium. There should be some equations for this
somewhere.
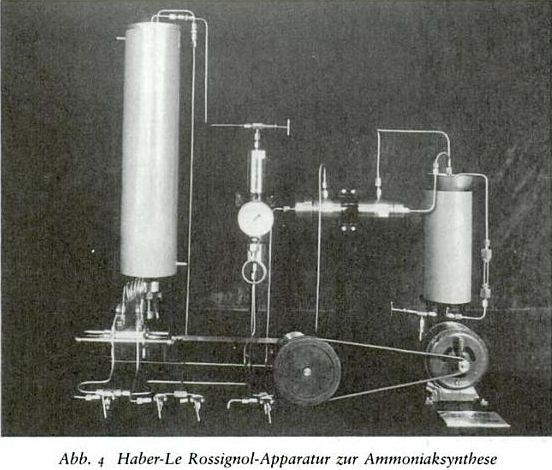
|
|
|
agorot
Hazard to Self
 
Posts: 73
Registered: 25-1-2010
Location: too fed-o-phobic to say :D
Member Is Offline
Mood: like an activated complex
|
|
So I've just finished reading The Alchemy of Air. It was very informative, but sadly, it doesn't look like the Haber process is going to be
performed in my house.
It seems that Haber was actually using 100-200 atm in his "little tabletop machine." The book says that this is pressure enough to crush most modern
submarines. That seems believable to me.
The reaction was carried out in "thick-walled quartz tubing encased in an iron jacket." This tubing was made from a full quartz crystal, and a drill
was used to hollow out a narrow cavity for the inner part of the tube, and then the outer part of the tube was made more cylindrical, and then it was
surrounded with the iron.
When Haber built is very first prototype, it ran at 1000C. In 1908 he made several improvements to the design to allow it to run at higher pressure,
so he only had to run it at 600C.
As for the catalyst:
| Quote: |
Haber tried powdered nickel, manganese, and platinum; none did the trick. So he turned to more exotic elements. One of the many consulting jobs Haber
had done involved looking for ways to make better filaments for electric lights, and the company he had worked with had supplied him with a number of
rare elements. He still had samples of many of them, and tried them in his ammonia machine.
In March 1909, one year after signing with BASF, Haber had a breakthrough. His team put a bit of one of his light bulb elements, a bluish black
brittle metal called osmium, in the high-pressure chamber, heated it, and ran hot nitrogen and hydrogen over it. The yield of ammonia shot up.
|
The only problem is that osmium was way too rare. BSAF could not have economically produced synthetic ammonia in this form. Eventually though, Haber
and Le Rossignol (another member of Haber's team) found that uranium also worked suitably well. It wasn't quite as good as osmium, but it was
close enough.
That's what I've found about Haber's original machine. I think to build something like that it would take a couple of thousands of dollars, not a
couple of hundred.
There is something else. Apparently, there was another chemist working for BASF, Ostwald (you may know the name from the Ostwald Process), that
thought he had beat Haber to it. He was using a strait iron catalyst, or so he thought. In reality, he was using some very high purity iron wire for
his experiments to make ammonia that had been used, and contaminated by, another chemist. His catalyst contained significant amounts of Iron Nitride,
and actually he had just been heating iron nitride in the presence of hydrogen to make ammonia.
Does anyone know how to make/obtain Iron Nitride--either the (II) or (III) salts? Incidentally, I think it can be made if you react iron with, you
guessed it, Ammonia.
[Edited on 13-5-2010 by agorot]
[Edited on 13-5-2010 by agorot]
|
|
|
agorot
Hazard to Self
 
Posts: 73
Registered: 25-1-2010
Location: too fed-o-phobic to say :D
Member Is Offline
Mood: like an activated complex
|
|
Quote: Originally posted by S.C. Wack  | | Quote: | | They first demonstrated their success in the summer of 1909, producing ammonia from air drop by drop, at the rate of about a cup every two hours.
|
Did this happen to feature osmium?
Or was it uranium?
[Edited on 13-5-2010 by S.C. Wack] |
From my sources, Haber was using Uranium at that point.
Another chemist named Mittasch, working with Bosch, found in late 1909 that a catalyst of magnetite and aluminum oxide and calcium. Now other
catalysts are available.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Steel is nitrided by a plasma/ion process. Not much better than Birkeland-Eyde, I'm afraid.
Tim
|
|
|
agorot
Hazard to Self
 
Posts: 73
Registered: 25-1-2010
Location: too fed-o-phobic to say :D
Member Is Offline
Mood: like an activated complex
|
|
Nitrides
Lithium, specifically.
Lithium reacts with nitrogen in the air to form Lithium Nitride very quickly. It is a black compound. Lithium Hydroxide and Lithium Oxide are both
white compounds. You know that lithium nitride forms much more quickly than the latter two compounds if you've ever cut a piece of lithium in half. It
turns black quickly, although some lithium oxide and eventually hydroxide is formed. Then this happens:
(A) Li3N (s) + 3 H2O (l) → 3 LiOH (aq) + NH3 (g)
If only there was some way for this to happen:
(B) 2 Li3N (s) + 3H2 (l) → 6 Li (s) + 2 NH3 (g) <--NOTE: doesn't occur
I got really excited just a minute ago because I was thinking that you could possibly use equation (B), but then I did some research and supposedly,
this one happens:
(C) Li3N (s) + 2 H2 (g) → LiNH2 (s) + 2 LiH (s)
Theoretically, if you could get equation (B) to work, instead of equation (C), your lithium would not be used up except for efficiency losses (your
lithium would react with oxygen in the air or something if you had no way to purify nitrogen first to make lithium nitride).
The only other problem would be to deal with this equation:
(D) 2 Li + 2 NH3 → 2 LiNH2 + H2
| Quote: | | Lithium amide reacts vigorously with water to generate gaseous NH3. In experiments at Argonne National Laboratory, in which it was mixed with water
and stirred at room conditions, about 23 percent of the theoretical yield of NH3 evolved as a gas in the first 0.6 minutes |
from http://cameochemicals.noaa.gov/chemical/991
(E) LiNH2 + H2O → LiOH + NH3
(F) LiH + H2O → LiOH + H2
Equations (E) and (F) could be used if equations (C) or (D) were to occur so you would end up with lithium hydroxide.
Unless you could find a way to very efficiently decompose lithium hydroxide (melting point ~460C) into lithium metal, this process would not work well
for a home chemist.
So there are two ways to make lithium work efficiently:
1) Find a way to reduce lithium hydroxide to metallic lithium efficiently
2) Find another metal that is CHEAP that acts as lithium does in that it reacts with nitrogen at atmospheric pressures and temperatures (or slightly
above within reason), and you don't care if you have to use a lot of it because you are just going to end up with it's hydroxide and concentrated
ammonia. That way you just use up a lot of that metal. Lithium is too expensive in my opinion to be used for obtaining large amounts of ammonia, but
perhaps small amounts.... You will get less than a gram of lithium from a lithium battery, and they are not too cheap.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
"Lithium is too expensive in my opinion to be used for obtaining large amounts of ammonia, but perhaps small amounts.... You will get less than a gram
of lithium from a lithium battery, and they are not too cheap. "
You get more then a gram of Li from batteries. Ill have to look at my notes but I think its around 1.2grams per battery. And any amount of ammonia
from Lithium is to expensive considering Ammonia is in no way hard to make.
| Quote: | The only other problem would be to deal with this equation:
(D) 2 Li + 2 NH3 → 2 LiNH2 + H2
|
This is no problem. Your reacting Li3N with H2 to make LiNH2 an LiH, there is no Li present at the formation of Ammonia. Your reacting this with H2O
to generate the NH3. All extremely inefficient. Not to mention that Li + NH3 only forms LiNH2 after reacting with an alkene or heated, all requiring
added expense.
To add more insult to injury wouldn't the theoretical reaction B occure on electrolysis of the melt(If you can get a melt) formed from the Nitride and
H2. Sorry to stray further off topic but would Li Dissolved in Liquid NH3 and electrolysis performed cause the Lithium to act as a catalyst when N2
and H2 is the feed? Just curious as to why or why not.
[Edited on 14-5-2010 by Sedit]
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Why use rare and expensive Li for such a purpose? You could just as easily use Na, although it does not slowly combine with N2 like Li, but instead
reacts very quickly with it, and may possibly be induced to burn in it. You could also use K, which spontaneously ignites in N2, and besides K3N, may
also form a pernitride K2N2 and supernitride KN2. However, with Na and K, you would have to safely contain the reaction somehow because of the large
amount of heat rapidly generated.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Given that the quote I included from Model Catalysis of Ammonia Synthesis..., and references papers by Haber and Mittasch, I'll go with
osmium being Haber's catalyst used in his demonstration in 1909, and Mittasch's development of the iron catalyst an outgrowth of BASF's interest in
Haber's work and Ostwald's early discovery that hadn't panned out. The demo ran at 175 bar.
The ceramic in the Euro patent isn't porous, it's a Proton Conducting Ceramic - a subset of ionic conductors. In the device hydrogen is transferred
through the ceramic as ions - protons - and delivered to the other side where it is converted back to hydrogen; depending on the electrode on the
surface it can be effectively a lightly adsorbed hydrogen atom.
The use of lithium as a nitrogen to ammonia converter is close to the electrolytic method in one of those papers I uploaded. In that process a molten
salt bath (NaCl-KCl-Li3N) is used, N2 is reduced to N<sup>3-</sup> at the cathode, the nitride migrates through the salt electrolyte to
the anode where it reacts with the hydrogen migrating through the porous metal electrode. It looks to be at least as energy efficient as the H-M-B
process using iron catalysts, doesn't involve messing about with elemental lithium outside of the original Li3N formation to make the electrolyte, at
higher temperatures can use nickel electrodes.
Two patents on the process, IMHO PPP examples, for much detail one would have to chase down the journal papers referenced in the patents.
Attachment: 6881308_Electrochemical_synthesis_of_amm.pdf (122kB)
This file has been downloaded 1556 times
Attachment: 7314544_Electrochemical_synthesis_of_amm.pdf (217kB)
This file has been downloaded 1025 times
An updated paper to one of the solid state NH3 synth papers I included before is at http://www.energy.iastate.edu/Renewable/ammonia/ammonia/2008... at 4+ MB too large to attach here.
--------------------------------------------
From Technology and Manufacture of Ammonia (Strelzoff):
| Code: |
At 300 C using promoted iron catalyst
pressure(atm) % NH3 in exit gases
100 8%
300 18%
500 27%
1000 47%
2000 65%
at 450 C
atm % NH3 in equilibrium
1 0,209%
10 ~2%
31.6 ~6%
100 ~16%
|
so it looks as if using iron based catalyst at pressures below 200 atmospheres gives pretty low conversion, and pressures much under 100 atm too
little NH3 production to be practical. Ruthenium based catalysts are more active, by that ref I gave earlier down to 100 bar and maybe lower. There's
also hope that variants of iron catalysts might work that low.
---------------------------------------------
@Polverone - yeah, been into it for a few years I was Skypeing with an old friend, who brought in a chum of theirs who turned out to be connected
withe several people very active in the stranded resources activities in North America.
Unfortunately, like far too many other renewable energy projects in the USA, the players seem to be more into PR and playing their cards close to
their vests, rather than actually publishing real data. Nocera and the magic Co electrolysis catalyst, NanoSolar's undisclosed details, EEstore and
the eternally slipping deadline, Aptera's not-yet-in-production-but-gamechanging BEV, there's so many 'breakthrough' companies out there that seemed
to be able to make it through another round of ventural capital raising even as another deadline goes by or promissed specifications fail to appear.
There's a lot of interesting stuff out there that does get published. Using the nitrogen fixing system from cyanobacteria in a stabilizing matrix for
NH3 synth, feeding eletricity to Methanobacterium palustre to convert CO2 to CH3; possible routes to making fuels and storing peak power.
Wind power keeps creeping down towards the cost of coal power, photovoltaics are seeing developments on a number of fronts.
As for interfacing the newer ways of making NH3 to variable supplies of power - the problem is a bit like using SOFC for power generation What I've
heard includes making the reactive elements in very small form to decrease thermal stresses, and either "banking back" subsets of the entire system to
run at/use fuel at the lowest level in order to keep the cells hot enough, or cycle through running subsets of cells while the remainder slowly cools
down, but becomes the active suset often enough to avoid needing a warm-up sstage.
|
|
|
agorot
Hazard to Self
 
Posts: 73
Registered: 25-1-2010
Location: too fed-o-phobic to say :D
Member Is Offline
Mood: like an activated complex
|
|
Quote: Originally posted by Sedit  |
And any amount of ammonia from Lithium is to expensive considering Ammonia is in no way hard to make.
|
That's why I specifically said the only way to make this process efficient is to use a very cheap metal that is easy to obtain. At the very beginning
of the thread, I specifically acknowledged that ammonia is easy to obtain from a strong base and an ammonium salt, but the whole purpose of
this thread is to explore other options and (maybe) find another way that is also somewhat cheap to use the nitrogen in the air. Because of
the availability of cheap ammonium salts, I don't think it will be easy to find a substitute for just making it with strong bases, but it is getting
more and more difficult to find raw alkali-metal hydroxide. It is almost always mixed with something.
I'm not at all insulted Sedit, and I hope you wouldn't think that I would be. The whole purpose of this forum is for people like us to come together
to talk about these ideas. I posted that equation you were referring to just so others could have it in mind when thinking about a possible lithium
(or other metal) based system.
@JohnWW, you said: | Quote: |
Why use rare and expensive Li for such a purpose? You could just as easily use Na, although it does not slowly combine with N2 like Li, but instead
reacts very quickly with it, and may possibly be induced to burn in it. You could also use K, which spontaneously ignites in N2, and besides K3N, may
also form a pernitride K2N2 and supernitride KN2. However, with Na and K, you would have to safely contain the reaction somehow because of the large
amount of heat rapidly generated. |
I didn't say we use lithium, in fact, this is what I said the following in my last post
| Quote: | So there are two ways to make this work efficiently:
1) Find a way to reduce lithium hydroxide to metallic lithium efficiently
2) Find another metal that is CHEAP that acts as lithium does in that it reacts with nitrogen at atmospheric pressures and temperatures (or slightly
above within reason), and you don't care if you have to use a lot of it because you are just going to end up with it's hydroxide and concentrated
ammonia. That way you just use up a lot of that metal. Lithium is too expensive in my opinion to be used for obtaining large amounts of ammonia
|
Sodium and potassium aren't much cheaper though... I was hoping for something like zinc or aluminum or iron or copper or nickel, but I haven't done
any research on these metals' nitrides and how difficult they are to make.
[Edited on 14-5-2010 by agorot]
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
Where's the pressure-source ? What compressor ?
==> Maybe something can be constructed by using a high-pressure waterjet-cleaner, letting it pump something that displaces the gases in a cylinder
...
==> 200 bar means: 1 liter of air at standard-conditions get's compressed to 5 or 6 ccm ... 
200 Bar will be held by a small-diameter glass-tubing; anyone can try that out by melting in some water and heat the ampoule safely in a furnace,
measuring the temperature ... until ist burst's (or not) : From this temperature the pressure can be calculated ...
Mineralogists do high-pressure exp. regularly: The ampoule holds the pressure, but is inserted in a steel-autoclave for safety ...
==> From the filling-level of the ampoule (%) and the temperature the pressure is known ...
==> Thats how eg. hydrothermal conditions are simulated ...
==> The steel-autoclave gets some water-filling too, to build up a counter-pressure, so the ampoule does get some assistance for containing the 500
Bar it regularly will have ...
==================
[Edited on 14-5-2010 by chief]
|
|
|
| Pages:
1
2 |
|