| Pages:
1
..
7
8
9
10
11
..
20 |
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Metabisulfite hydrolyses upon addition of water: Na2S2O5 + H2O => 2NaHSO3
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
I was going to do the silver mirror, but then I realized that I ran out of nitric acid to make silver nitrate with. And I left this big one-ounce
silver coin I have at my girlfriend's house. So I'd like to try that test at some point, but I think the reaction with bisulfite would be proof
enough. I finally just ordered some online because my stuff wasn't behaving, so I'll do that once it gets here.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
You could also form the adduct with 2,4-DNPH, and take a melting point. IIRC, there are tables published in Vogel for such crystalline derivatives.
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
Thx DJF90!
Melgar for your silver mirror tests you do not need to make silver nitrate, just use your silver coin as an anode in KNO3 solution, you will be amazed
how fast the silver turns into Ag2O, then dissolve it in aq. NH3!
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
I finally made some acetaldehyde in pure form!
I used the setup that I posted in my previous attempts, but this time I drew it for better understanding, sorry for the shitty line leading!
The glass tube was filled with copper oxide pressed into small granules of about the diameter of 3mm. Copper oxide was made with anodic oxidation, as
it seemed to me that this way the oxide is extremely fine.
At the startup I gradually increased the temp of the khantal wire (setpoint of TC1) to 500°C, but the rate of acetaldehyde evolution is also
acceptable at 400°C so I decreased the temp to 450 and kept at that. (the acetaldehyde is decompose @400°C to CO and CH4 but this was external temp
control, so the temepature in the tube during operation is surely below 350C or so I think...)
It should be noted that I melted a small capillary, made of glass that protrude into the glass tube and tried to control the temperature that way
(TC2), but as it turned out it was a bad idea and totally unnecessary. And I have not got a temerature controller with such a high response that this
layout would require.
I directly avoided the heating of the catalyst, so the heating element(Khantal wire) and the catalyst bed was separated (Heating part~15cm, catalyst
bed~15cm).
Identifying tests:
1 I led the gases into cc H2SO4! which was first get a yellow colour then got hot and charred after about 20min.
2 I led the gas into ammonical AgNO3 and heated it, a solid black precipitate was formed.
3 I led the gases into ccNaOH, first it got a yellow tint then turned into red and a tacky melted polymer with an apple like smell settled on the
bottom.
4 The gas absorbs in aq ammonia and the solution on drying give a crystaline compound.
5 The NaOCl solution gets warm and turns yellow upon absorbing the gas.
6 If you drop some ccH2SO4 into the condensate (pure acetaldehyde) the condensate immediately starts boiling and if you do not stopper the bottle
immediately you only get char, othervise you get two layers, a black layer at the bottom, and a yellow-white layer above it, which smells like
pinewood three?!
7 I also lead the gas into Ca(OH)2 with HCHO followed the org synt volt 2 instructions, and after the required weight increase of the reaction mixture
I poured some additional HCHO solution into it, (because the HCHO smell was totally disappeared after the addition), and got a yellow colour, which
after keeping the mixture @ 45°C for an hour turned intense red. and a fine yellow powder settled at the bottom. (I did not have the time to isolate
the product.....)
It seems to me that I forgot something, but I always have this feeling, anyways
So based on this reactions the dehydrogenation mehod, which is noted for lower by products, works at home, and can be reproduced by anyone..
After the tests, the setup was being run for additional 12 hours, during which I collected 69g of acetaldehyde, I poured it into a vial with a srew
cap, upon opening the vial the liquid began to boil which is not literally means boiling, If you open the vial and follow a dust particle with your
eyes you can see that it goes up and down, and a condensate on the vial walls can also be observed, furthermore on every opening of the vial I heared
a 'shssssss'.
Which amazed me is that after the refrigerator there was still a lot of acetaldehyde uncondensed, I do not know how much, I can only say that the best
is to use it as is, I am sure that I do not bother condensing it next time...
I will make some pentaeritriol and modify the setup to make acetic acid from ethanol with car exhaust catalyst (Pt on Al2O3) in my next project, wich
I wanted for a long time, and after this success I seems to me that it wont be that hard
Any comments or improvements would be appreciated!
Attachment: acetaldehyde exp setup.rar (34kB)
This file has been downloaded 1095 times
|
|
|
AceParkle
Harmless

Posts: 1
Registered: 2-5-2010
Member Is Offline
Mood: No Mood
|
|
| Quote: |
| Quote: | | While researching refractory materials I came across a high fired granular porous clay material which is used as an artificial soil for aquatic
plants in decorative ponds and aquariums , as a substitute for ordinary gravel , and it occured to me that this might make a useful carrier for a
catalyst . A similar material is a porous alumina which is used in water filtration as a substrate on which grow beneficial bacteria and algae , and
this ceramic could be broken up into granules using a hammer , and the granules could also be useful as a porous substrate for carrying a finely
divided catalyst . |
|
I absolutely agree with your statement Rosco Bodine. Just like what I did and observed using the water filtration. It proves that this is very helpful
carrier for a catalyst.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
I did the bisulfite adduct on some of the distillation product from my fermented sugar water. After a day or so, there was a fine precipitate on the
bottom of the container, and the smell I recognized was gone. The trouble is, whenever I do a bisulfite adduct reaction, I always get just a tiny bit
of the adduct, even if there's quite a bit in there. Here's what I do:
1. Add some sodium metabisulfite to denatured alcohol and wait for it to dissolve. Keep adding until no more dissolves.
2. Add the aldehyde solution to the metabisulfite solution. (metabisulfite solution is about 2/3 the total volume)
3. Wait for adduct to precipitate.
I'm not sure if I should just add bisulfite as the adduct falls out or what. And as I understand, metabisulfite needs water to turn into bisulfite.
Maybe it takes a while for this to happen? In any case, it doesn't seem like you can actually find bisulfite that isn't metabisulfte, and if you
order bisulfite, they send you metabisulfite. Also, I'm not sure what the adduct solubility is like. It seems to dissolve in methanol, but fall out
in ethanol and isopropanol. Maybe it's different depending on the aldehyde. I've been testing with vanillin, since I can add a known amount of
aldehyde and see how much precipitates, but I'm sure the other functional groups contribute to solubility.
Anyway, once I figure out how to do this reaction, I can quantify how much acetaldehyde I'm getting from fermentation.
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
I also tried to coprecipitate the acetaldehyde with NaHSO3 but found it impractical, the NaHSO3 (Na2S2O5+H2O) is expensive and it is not really like
Na2CO3+Ca(NO3)2, but for quantitative analysis purposes it may be usefull..
For purification of aldehydes see: http://www.sciencemadness.org/talk/viewthread.php?tid=10256#... Len1 post -Purification - stage 3- it contains a lot of usefull information.
I would distill off some liquid into cold ethanol out of the fermantation pot, an let is sit in a stoppered bottle filled with air under the sun for
about a week, and smell it, if you cannot smell acetic acid, you only made very tiny amount of acetaldehyde.
I also did this experiment, but I poured some acetaldehyde into a prechilled PET bottle stoppered it and left it outside for about a week, the
completion of the reaction could be seen on the shape of the bottle, after the addition of the acetaldehyde and closing of the bottle, the pressure
increased inside it for about 0,5-1 bar, and after about a week the bottle was dented, upon opening it a strong smell of acetic acid could be smelled.
It should be noted however, that acetaldehyde is very volatile, when I tried to condense it @-20°C only part of the acetaldehyde condensed, despite
of the very efficient chilling (the venting tube after the refrigerator was frosty) when I took a nosefull of the gases of the venting tube, the smell
was still strong! Also, if you only notice an apple like smell, you only made ppm of acetaldehyde, It has a pungent odour, comparable with
formaldehyde, but unlike formaldehyde its smell is pungent immediately after a nousefull of it..
|
|
|
Mildronate
Hazard to Others
  
Posts: 428
Registered: 12-9-2009
Member Is Offline
Mood: Ruido sintetico
|
|
I do acetaldehyde synth yesterday in mybody from vodka 
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
I remember the one girl I was dating once worked in a refugee center for mostly burmese refugees. The one Burmese guy that worked with her always had
this strong smell about him that I couldn't place. Now I realize it's acetaldehyde. Guess he must have been quite the lush...
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
I did two more experiments to make acetaldehyde
I electrolysed copper onto khantal wire, then heated it in air to oxidize the plated copper into CuO then placed the wire into a ketene generator (the
setup was the same described in organic reactions), first the setup was running at dull read heat, then heated I presume the maximum that the khantal
wire is able to resist to.
At the max temp, there was a lot of gas generation, it was so much that i could lit it and the continuous flame was about 10cm long.
So I can state that this is the fastest and easiest method to genererate a shitload amount of acetaldehyde!
If I have time in work, I will take a HS-GC-MS spectra from each pure sample collected at the two temperatures, and from the first, made by pressed
CuO catalyst.
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Without some isolation of the gas, you can't really say whether this was acetaldehyde. At higher temperatures (450+C) formaldehyde might predominate,
or even smaller molecules. That said, I'm sure that if you can control temperature and flow rate adequately you should be able to generate useful
quantities using your setup.
|
|
|
Globey
Hazard to Others
  
Posts: 183
Registered: 9-2-2009
Member Is Offline
Mood: No Mood
|
|
I don't know if it has been repeated upon, but alanine (simple amino acid) and not beta alanine I believe, will react with dilute HTH to form
acetaldehyde. One might also want to consider just using a virgin catalytic converter, and dripping Everclear through that, at room temp, and having
a condenser hooked up. The hexavalent chromium/dichromate reactions to me are really not so elegant, and I always like to stay away from anything
polluting. Always go green if you can. Just my opinion. It's more elegant, and it's also the right thing to do.
|
|
|
john_smith
Harmless

Posts: 1
Registered: 4-6-2010
Member Is Offline
Mood: No Mood
|
|
I just want to give you a way to make acetaldehyde. A very simple one is by heating a mix of Calcium acetate + Calcium formate. Both calcium salts
are very simple to make.
The reaction is the following
Ca(HCOO)2 + Ca(CH3COO)2 ----> 2CH3CHO + 2CaCO3.
[Edited on 4-6-2010 by john_smith]
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Hm, yes. But have you actually tried this? I don't doubt that some acetaldehyde does get produced (I see for example http://www.nature.com/nature/journal/v165/n4193/abs/165402a0... supports your idea), but depending on temperature, heating rate, admixture and so,
different proportions of products would result. I recommend http://www.scribd.com/doc/28118713/Thermal-Decomposition-of-... , not for insight into this particular proposed way of making acetaldehyde but
just for some idea of the variety of possible reactions (and by extension the need for fractionation and workup).
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
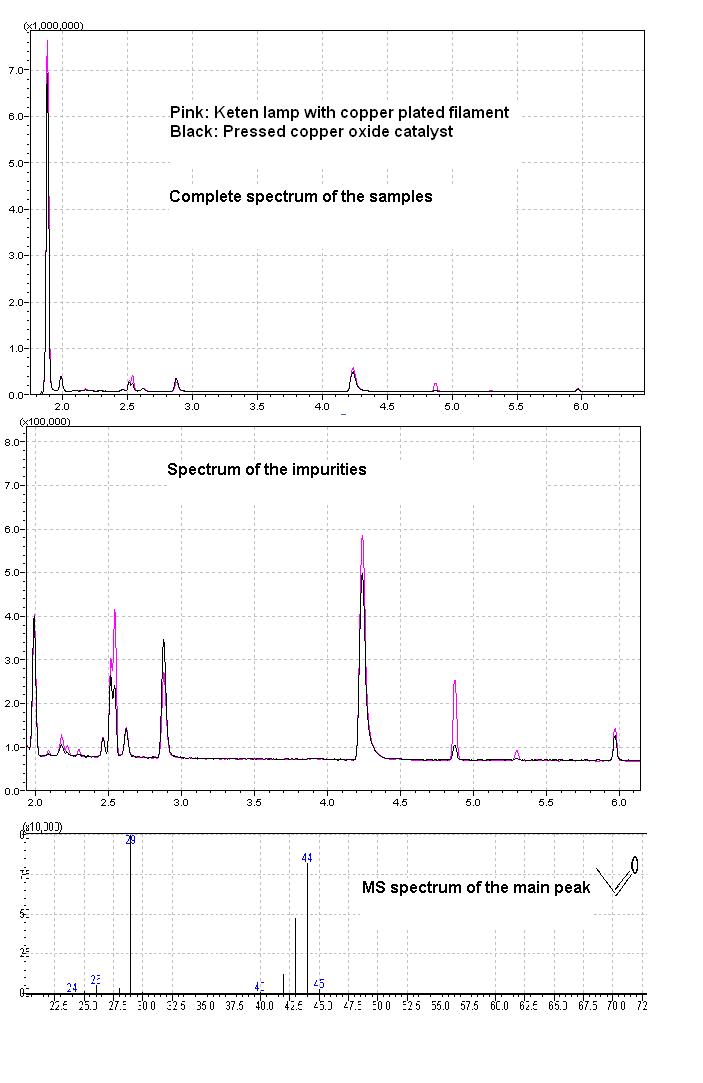
Here are the HSGC MS results of the two samples made by completely different apparatus.. to my deepest suprise.. the composition of the two samples
are identical.. eeh!!
5micro liter of the pre chilled stuff with a chilled syringe was injected to a HS ampoule and heated to 300°C the absorbing component of the GC tube
was of poly ethylene glycol.
The components on the main spectrum in ascending order of the retention time:
Acetaldehyde: 1,93 min.. I can say.. that lab quality acetaldehyde can be made at home which is just awseome 
Acetone: 2,17min
Butanal: 2,45min
Ethyl acetate: 2,513min
Diethyl acetal: 2,54min
Then: 2 butanon, ethyl alcohol, paraldehyde (4,8min), metaldehyde, dioxolane.
I would like to add that I used denaturated alcohol, which is mainly adulterated in my country with methyl ethyl ketone (2 butanone), that explains
the 2 butanone peak, the ethyl alcohol peak is evident
Also I would like to add that more acetaldehyde can be produced by the lamp method according to the results, but it can be attributed also to error of
measurements, I mean messing with prechilled 5micro litre syringe can really cause difference at paralell measurements.
More ethyl acetate is produced by the lamp method due to higher temperatures, also the amount of paraldehyde and the tetramer is more that I cannot
explain taking into consideration the increase in the amount of ethyl acetate, these impurities are only a few percent of the total sample though.
Based on the results both setups are recommended for a trial to every home chemist
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Actually, I tried mixing calcium hypochlorite pool shock and alanine (from nutrition stores) and I have to say, this has got to be the easiest way by
far to make small amounts of acetaldehyde. Look up "Strecker degradation" to see how the reaction works. I've also heard, though I can't verify it,
that TCCA pool shock will give the corresponding nitriles rather than aldehydes.
|
|
|
Ephoton
Hazard to Others
  
Posts: 463
Registered: 21-7-2005
Member Is Offline
Mood: trying to figure out why I need a dark room retreat when I live in a forest of wattle.
|
|
you are right there meglar TCCA makes nitriles from aminoacids very easily.
thankyou very much to both your self and jimmymajesty for pointing out another use for alanine
and a ketene lamp.
e3500 console login: root
bash-2.05#
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
JIMMY! Great experiment! I was going to suggest something similar, but don't have that kind of analytical equipment to hand. I've
seen people rabbiting on about the idea of building mini catalytic reactors but rarely hear about practical attempts and have never seen a spectrum
from one.
I'm kind of busy and can't read back to your other posts right now, but did you try depositing the copper onto zeolite for the cake? This is mentioned
all over the patents regarding copper catalysts, as well as how trace impurities in the copper affects the process. Like, I think zinc helps produce
MEK or butanal in the product, or something along those lines.
From memory, they use ?ZSM-10? zeolite as the support. The vast surface area the structure presents accelerates the catalysis. The specific pore size
seems to also influence the selectivity of the process.
Of coarse, the patents also discuss how they deposited the copper from solution (easy stuff). Google around "ethanol copper zeolite patent" and you'll
be underway in no time.
DCM, I've never seen bearing a flammable sticker in real life. I have a tank of paint stripper, a container of pure DCM for everyday
solvent cleaning and an up to date reagent grade bottle from a laboratory supplier. None of them have flammable stickers. But it has now been upgraded
to a potential carcinogen. Meaning it can have a skull and cross-bone sticker in place of it's old harmful cross. I bet all the older chemists aren't
impressed! I'm not! Hopefully it's not another benzene. I haven't seen these stickers on paint stripper yet, which is odd as they are very high
concentration sources of the solvent. They're still using the harmful sticker on both stripper and the reagent bottles.
I have a bone to pick with people constantly referring to things as toxic, when they're actually more like harmful. It seems anything with the
potential to cause any harm is upgraded to toxic now. Whilst things that do present real risks (like cancer) still have a harmful sticker on them.
Since irritant also exists, it makes me question precisely what harmful is still being used for by these people. To me, toxic means it creates a long
lasting risk at everyday exposures. So benzene would be toxic to me, as it damages DNA and creates a long lasting, carcinogenic risk. DCM would fall
into the same category for myself. Following that pattern of logic, I'd nominate DCM for a harmful cross (with harmful / irritant under it) and a
skull and cross-bones (with carcinogen under it), as it is both immediately irritating and slightly dangerous in terms of exposure but also capable of
longterm damage. Cyanide would be harmful, as (despite it being extremely dangerous) it does not accumulate or present a longterm risk. I would
seriously love for them to produce a new sticker visually indicating something is a suspect carcinogen, like a simple double helix (or to write this
under the skull and cross-bone, as they already do under the blanket, seriously outdated term poison). I would also like to see the NFPA 704 rating
system used on the containers. It's on the sides of the trucks, it should be on the containers people will actually be handling. I worry when I see
MSDS that take the safe route out and blanket list everything as dangerous to cover against liable action. It's like saying a relatively harmless drug
is in the same category as heroin. Causing users of the former to think the latter will be the same in terms of risk. Failing those changes to the
stickers, or in addition to them, I'd like to see an accumulation sticker to indicate whether acute exposure is going to build up. And I want all of
those changes regulated by someone other than the chemical companies, who will plaster them all over everything as the carcinogens start lining up.
Either way, what I'm saying is that I'm not happy with the level of information the quick references provide, and think it could easily be improved
upon. And students should be taught precisely what each of the few new variations means.
In terms of extracting DCM from paint stripper, get the cheapest, nastiest stuff you can. They usually have more DCM in them. It's usually around
50-70%. It will also be mixed with a polymer to make it thick enough to paint onto vertical surfaces without it immediately running off or evaporating
away. Before you distill it, wash it with some water. The water should start looking like someone's cum in it. There'll be opaque white goo floating
around in it (the polymer). Throw out the wash, distill the remaining organic. Using a saline wash may help prevent loss of DCM, as some of it will
end up in the aqueous phase using pure water. If you go straight to distillation with the polymer, you may cake it onto the flask. It boils like crazy
under only moderate vacuum, but it's also not going to liquefy at the condenser all that well, so it'll end up spitting out the pumps exhaust. Go
with atmospheric distillation given that it boils so close to body temperature and doesn't break down. It's a fairly 'fun' distillation given how easy
it is compared to oxygen sensitive, high BP things that bump under vacuum.
Bulk pyridines from a catalytic reactor? One thing that really interested me about this method of producing aldehyde was that the
product is part of the one pot pyridine synthesis (throw it all together, stir away). One can cheaply and easily ferment hundreds of liters of ~20%
alcohol at home in a week or two using raw sugar and distillers yeast. The excess water can be quickly and cost effectively stripped by dumping in a
bag of plaster I believe, then decanting from the cake. The remaining liquid can then yield 100% ethanol under a more reasonably sized vacuum
distillation (which breaks the azeotropic mix). You could also simply warm up the drum with an immersion heater (even the plastic ones will take the
heat) and gently strip the azeotrope off, then run it through a smaller volume vacuum distillation in the glass. Homebrewers of moonshine go as simple
as putting a plastic bucket of alcohol inside another plastic bucket with a lid and then warm up the alcohol containing bucket, letting the azeotrope
condense on the sides of the outer bucket. Catalytically produce aldehyde from the vacuum distilled azeotrope, feed it into the one pot. I wonder if
this same catalytic method works for methanol to formaldehyde (another component of the one pot). Yes, I know about the niacin method. But this seems
reasonably realistic if you wanted larger volumes of pyridine. Or, for myself, just for the fun of doing something involving both biology and
chemistry. I've brewed up huge volumes of alcohol using distillers turbo yeast and raw sugar in a big 210l plastic storage drum with an aquarium
heater. The drum was formerly used to internationally transport olives. Yum!  And even featured a very nice screw on lid that could easily be ported for an air pump, lock or vapor outlet; with
your condenser possibly being as simple as a roll of PB / PEX plastic plumbing pipe, secured to the lid using a push fit cold header tank connector
(they sell them specifically for this pipe and they feature a rubber gasket). I just loosely put the lid on and let it go. It was so tough I could
kick it around the floor and barely scratch it. It all cost next to nothing and took next to no effort. I 'sterilized' with a quick rinse of boiling
water. I rolled the culture up in a 15l container before hand, using more clean methods (e.g. boiled water), then inoculated the 210l of water at 50%
of the recommended sugar concentration, so as to avoid thermal death. Then poured in the remaining 50% once it had calmed down a little. I was using
25kg sacks of sugar from a bakery supplier (real, real cheap). The water in the bulk drum was straight from the tap. The rate of fermentation was
hilarious. You can also yield other useful building blocks, solvents and reagents from the process. It's like a total synthesis, but without the total
overkill. The appeal of going from something as simple as sugar, water and yeast is high for myself. In addition, yeasts can perform other interesting
feats of biochemical manipulation. And the raw product of this method is a highly valued resource for those who enjoy getting very drunk, very
quickly, very cheaply. Might I suggest sampling some 100% ethanol, or buying a nebulizer and blowing O2 through it at the same time (you could use the
pump that comes will a full kit for aerating the fermentation, not that it really needs it, but it might help churn up the mix). Caution, ethanol +
pure O2 + drunk people = extreme fire hazard. These methods would also be very easy to lay down at the small scale, starting from some off the shelf
alcoholics grade, no name vodka. The fermentation is bulletproof, it's about the oldest, most well studied biochemical method committed to text. You'd
yield around 40 liters of 100% ethanol from a 210l drum fermentation. And foaming isn't an issue at all given how simple the sugar supply is; it
doesn't foam. I also doubt you'll get into that much trouble, if any, distilling your own alcohol if you can demonstrate that it's not intended for
consumption (which is what the tax is there for). When it comes to chemicals, the law is more concerned with why you have it and how safely you can
handle it rather than the fact you have it in the first place. And even featured a very nice screw on lid that could easily be ported for an air pump, lock or vapor outlet; with
your condenser possibly being as simple as a roll of PB / PEX plastic plumbing pipe, secured to the lid using a push fit cold header tank connector
(they sell them specifically for this pipe and they feature a rubber gasket). I just loosely put the lid on and let it go. It was so tough I could
kick it around the floor and barely scratch it. It all cost next to nothing and took next to no effort. I 'sterilized' with a quick rinse of boiling
water. I rolled the culture up in a 15l container before hand, using more clean methods (e.g. boiled water), then inoculated the 210l of water at 50%
of the recommended sugar concentration, so as to avoid thermal death. Then poured in the remaining 50% once it had calmed down a little. I was using
25kg sacks of sugar from a bakery supplier (real, real cheap). The water in the bulk drum was straight from the tap. The rate of fermentation was
hilarious. You can also yield other useful building blocks, solvents and reagents from the process. It's like a total synthesis, but without the total
overkill. The appeal of going from something as simple as sugar, water and yeast is high for myself. In addition, yeasts can perform other interesting
feats of biochemical manipulation. And the raw product of this method is a highly valued resource for those who enjoy getting very drunk, very
quickly, very cheaply. Might I suggest sampling some 100% ethanol, or buying a nebulizer and blowing O2 through it at the same time (you could use the
pump that comes will a full kit for aerating the fermentation, not that it really needs it, but it might help churn up the mix). Caution, ethanol +
pure O2 + drunk people = extreme fire hazard. These methods would also be very easy to lay down at the small scale, starting from some off the shelf
alcoholics grade, no name vodka. The fermentation is bulletproof, it's about the oldest, most well studied biochemical method committed to text. You'd
yield around 40 liters of 100% ethanol from a 210l drum fermentation. And foaming isn't an issue at all given how simple the sugar supply is; it
doesn't foam. I also doubt you'll get into that much trouble, if any, distilling your own alcohol if you can demonstrate that it's not intended for
consumption (which is what the tax is there for). When it comes to chemicals, the law is more concerned with why you have it and how safely you can
handle it rather than the fact you have it in the first place.
[Edited on 20-6-2010 by peach]
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
I requested a reference for this in the references section. You need pyridine tho 
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
See! Everyone loves pyridine! 
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by peach  | | I have a bone to pick with people constantly referring to things as toxic, when they're actually more like harmful. [...] Cyanide would be harmful, as
(despite it being extremely dangerous) it does not accumulate or present a longterm risk. |
Under this theory,
cyanide would be non-toxic. Because this is the internet, I will point out explicitly that this is absurd. Just as you don't get your own facts in an
argument, you don't get your own language in public discourse. That is, unless you are in discourse with Tweedledee and Tweedledum; with them you can
say anything you like.
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
I also read patents how to make catalysts that for example increase the ethyl acetate yield, but I think the only product of value of catalytic
dehydrogenation of ethanol is acetaldehyde, if I were to make ethyl acetate I surely will not make it this way.
The making of some hundred of pressed copper oxide pellets was really a pain. I was glad when I finished, that I will not have to in my rest of my
life. I have the shivers of the simple thought of a 4%Zn 6%Mn 10%Co 80%Cu homemade catalyst, but If you have the time to make it I will be happy to
try it out
I took a photo about the CuO catalyst, (before and after shots)
I also took some catalyst pellets after a 12 hours of run and broke them to show that also the inside parts are reduced, so It must be porous.
Attachment: CuO catalyst.doc (96kB)
This file has been downloaded 976 times
[Edited on 21-6-2010 by Jimmymajesty]
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
It'd be classed as harmful, since it neither accumulates or causes permanent damage; it actually burns off quickly compared to other chemicals.
Alcohol and tobacco (do they have a chemically additive sticker?  ) are both
harmful and toxic at levels that are used everyday by vast numbers of people. They are also both flammable, alcohol for obvious reasons and cigarettes
because they contain burn enhancers. They are responsible for hundreds of millions of deaths and they lack all three labels. As is table salt lacking
it's harmful / toxic sticker due to the heart disease it causes when exposure is at realistically prominent, everyday excesses. ) are both
harmful and toxic at levels that are used everyday by vast numbers of people. They are also both flammable, alcohol for obvious reasons and cigarettes
because they contain burn enhancers. They are responsible for hundreds of millions of deaths and they lack all three labels. As is table salt lacking
it's harmful / toxic sticker due to the heart disease it causes when exposure is at realistically prominent, everyday excesses.
| Quote: |
The making of some hundred of pressed copper oxide pellets was really a pain. I was glad when I finished, that I will not have to in my rest of my
life. I have the shivers of the simple thought of a 4%Zn 6%Mn 10%Co 80%Cu homemade catalyst, but If you have the time to make it I will be happy to
try it out |
Could you not skip the pelleting by depositing it onto a granular zeolite? Which'd also increase your surface area by orders of magnitude.
[Edited on 24-6-2010 by peach]
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
The reference now in the RRthread speaks of using DILUTE HNO3 to generate an aldahyde from an alcohol. Mainly they speak of Benzaldahyde from BnOH but
it could be adapted rather well for acetaldahyde. Im currently working with it to oxidise all the was to AcOH but the smell of Acetaldahyde is very
very strong. After a period of ageing the mix one could just distill off the aldahyde or precipitate as the adduct.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
| Pages:
1
..
7
8
9
10
11
..
20 |