| Pages:
1
2
3
..
6 |
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Nitromethane, nitroethane, nitropropane synthesis via industrial route
Nitroparaffins like nitromethane, nitroethane, and the two isomers of nitropropane are produced industrially by reacting HNO3 and propane at about 150
psi and 400 degrees C. Now, these two reagents are far more easily obtained than the ones usually referred to for lab-scale syntheses, the only
problem seems to be the specialized equipment needed. Still, this temperature and pressure combination is by no means beyond the capability of a
homemade device built from stainless steel tubing. Has anyone looked into building something that could do this? If not, I'm thinking I could give it
a try in the near future.
My plan for such a device consists of a propane torch tank, which would bubble propane through heated nitric acid. This pressurized mix of nitric
acid and propane would then be fed into a heated coil of stainless steel tubing. At the end of the coil, there would be a manual valve, which would
send the outflow through a condenser and into a collection flask.
Nitric acid/propane ratio would be controlled by changing the nitric acid temperature. Overall pressure would be controlled by warming or cooling the
propane tank. Flow rate would be controlled by the manual valve. Everything after the nitric acid flask would be made out of 304L stainless steel,
which is more or less impervious to concentrated nitric acid.
So, any chance of success here? What do I need to worry about? How exothermic is this exactly? I plan on using fairly narrow tubes, as they'd be
able to withstand much higher pressures, plus in the event of some explosive-type reaction, there wouldn't be much in the tubes anyway.
Attached is a pdf detailing the industrial route.
Attachment: nitroalkanes.pdf (214kB)
This file has been downloaded 10001 times
[Edited on 4/12/10 by Melgar]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
To me, this reads like a recipe for disaster - you will not have the kind of neccessary control found in an industrial set-up and this could easily
lead to a serious accident at some point.
Pick something safer, like sword-swallowing?
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
You do not cite a reference for the reaction, and as such this thread should have been opened in beginnings, as the moderators keep insisting.
However, I would like to comment on what you have written.
| Quote: | | Nitroparaffins like nitromethane, nitroethane, and the two isomers of nitropropane are produced industrially by reacting HNO3 and propane at about 150
psi and 400 degrees C |
I seem to recall the reaction being performed in the vapour phase at 450*C, with no mention of pressure (ref. IIRC is Functional groups -
reactions and interconversions by Denis G Meakins, OUP).
| Quote: | My plan for such a device consists of a propane torch tank, which would bubble propane through heated nitric acid....
Everything after the nitric acid flask would be made out of 304L stainless steel, which is more or less impervious to concentrated nitric acid.
|
For a start, I wouldn't use a propane tank to heat ANY acid. I'm fairly certain these are made of mild steel or the like; something concentrated
nitric acid would probably make short work of at room temperature. Bear in mind that although 304L may be resistant to conc. nitric acid at room temp,
nitric acid vapours at 400*C and 150psi are a completely different beast. Be sure before you do it, although I thoroughly advise you against it.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by DJF90  | | You do not cite a reference for the reaction, and as such this thread should have been opened in beginnings, as the moderators keep insisting.
However, I would like to comment on what you have written. |
Sorry, I have been reading about this reaction so much lately I forgot it isn't common knowledge. I edited my first post to include a reference.
| Quote: | | I seem to recall the reaction being performed in the vapour phase at 450*C, with no mention of pressure (ref. IIRC is Functional groups -
reactions and interconversions by Denis G Meakins, OUP). |
See the link I posted. And yeah, I'm well aware it's a vapor-phase reaction.
| Quote: | | For a start, I wouldn't use a propane tank to heat ANY acid. I'm fairly certain these are made of mild steel or the like; something concentrated
nitric acid would probably make short work of at room temperature. Bear in mind that although 304L may be resistant to conc. nitric acid at room temp,
nitric acid vapours at 400*C and 150psi are a completely different beast. Be sure before you do it, although I thoroughly advise you against it.
|
I may not have been clear in my description. The propane tank would never be exposed to nitric acid, its job would merely be to pressurize the
reactor with propane. I haven't had much luck finding high-temperature resistance charts for stainless steel and nitric acid. There does seem to be
significant corrosion at high temperatures, although I'm not sure if that can be reduced by changing the concentration, or if that applies to liquid
or vapor or both. Also, that corrosion is measured in mm/year, so I'm thinking a little corrosion might be acceptable. However, titanium would
definitely work very well for this, it's just expensive.
I do have a degree in mechanical engineering, so I'm not totally clueless. As far as the reaction area, (ie, the heated part) I'm thinking molten
zinc would keep it at the right temperature. If I did actually build this thing, I'd definitely build some sort of enclosure around it to contain any
mechanical failures. Still, nitric acid vapor is preferable to liquid nitric acid as a reactant, since it's a lot less concentrated. The liquid HNO3
container would be in a protected spot off to the side, where any problems in the reactor area wouldn't affect it.
|
|
|
Z8320
Harmless

Posts: 7
Registered: 13-12-2009
Member Is Offline
Mood: No Mood
|
|
I'd like to suggest a safer route that doesn't seem to have been suggested before on this forum(or on any others I've seen.
1)Self Condensation of Nitromethane to methyl nitroacetate as depicted in the link below http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv6...
2)Deprotonation with sodium methoxide of methyl nitroacetate and then nucleophilic attack of this on chloroacetate or bromoacetate which can be
readily made from glycine by diazotization in the corresponding mineral acid. I suspect the rather exotic solvent used in this reaction can be
replaced with any other solvent that won't be deprotonated by the sodium methoxide. I'm not even sure why it can't be done in methanol alone. The
benzene also sounds unnecessary, seeing as it's only used in the extraction step. You could probably even figure out a way to improve on their
relatively shitty yields.
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv6p0503
3)Decarboxylation of the resultant product is the only part I don't have references for, I just suspect it can be achieved relatively easily with the
proper application of heat and base.
For the preparation of alpha halo carboxylic acids from amino acids:
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv8...
For the preparation of nitromethane from chloroacetate: http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv1...
I suspect the above two links could also be used in conjunction to obtain a variety of nitroalkanes, though they too suffer in their yields a bit
unless you use a bromocarboxylic acid; there's a patent for that, but I'm not going to dig it up unless anyone cares.
|
|
|
thereelstory1
Harmless

Posts: 8
Registered: 12-4-2010
Member Is Offline
Mood: No Mood
|
|
Melgar,
There is an easier synthesis using sodium nitrite, I believe. If you look in organic synthesis.org you will find it.
|
|
|
Z8320
Harmless

Posts: 7
Registered: 13-12-2009
Member Is Offline
Mood: No Mood
|
|
I realize this is a bit off topic from the relatively suicidal and difficult to achieve industrial reaction that probably won't have much better
yields without some complicated gas recirculation, but there's little to suggest this will be easier than buying some simple reaction and distillation
glassware and basic reagents.
I've tried any number of gas phase reactions using my tube furnace, and they're in general best avoided due to very low yields, explosion risk,
consumption of time and money, and the inconvenience of controlling the pressure built up by the reaction. It's difficult to achieve stability even
with PID controllers adjusting your temperature and pressure.
[Edited on 4/13/2010 by Z8320]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Z8320 - You won't decarboxylate 2-nitrosuccinate to nitroethane. Only the carboxylic group to which the nitro group is alpha can be removed readily.
thereelstory1: You obviously didn't read the first post. | Quote: | | Nitroparaffins like nitromethane, nitroethane, and the two isomers of nitropropane are produced industrially by reacting HNO3 and propane at about 150
psi and 400 degrees C. Now, these two reagents are far more easily obtained than the ones usually referred to for lab-scale
syntheses, the only problem seems to be the specialized equipment needed. |
That would incorporate the use of nitrite don't you think?
|
|
|
gsd
National Hazard
   
Posts: 847
Registered: 18-8-2005
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Melgar  | | Nitroparaffins like nitromethane, nitroethane, and the two isomers of nitropropane are produced industrially by reacting HNO3 and propane at about 150
psi and 400 degrees C. ............................... |
@Melghar
Check this paper. It talks about the reaction being conducted at atmospheric pressure.
gsd
P.S. If I am not mistaken, this topic is also covered in groggins.
gsd
Attachment: The Gas-phase Nitration of Alkanes.pdf (1.8MB)
This file has been downloaded 2296 times
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
I'll post a quick sketch of the proposed reactor, since the design isn't exactly obvious. The whole thing would be behind thick plexiglass, in case
there's some sort of nasty reaction, although the narrow tubing should prevent too much from reacting at a time. The pressure is controlled by
changing the propane tank temperature. Initially, it would be best to have diluted HNO3, as that would slow down the reaction.
Also, I got offered a deal on .25 inch titanium tubing, and I went for it. So looks like l'm getting set to actually build this thing. And FYI, I am
a mechanical engineer, so I really am qualified to design stuff like this. I would discourage anyone else from building a contraption like this too,
however I have a piece of paper that says I know what I'm doing. :p
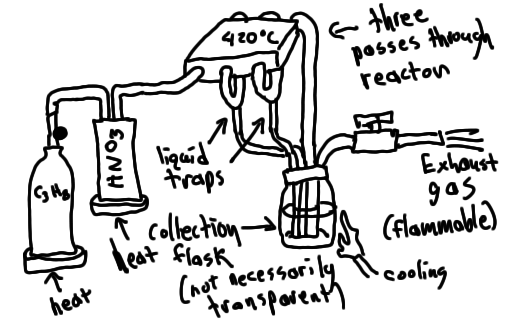
|
|
|
Z8320
Harmless

Posts: 7
Registered: 13-12-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DJF90  | Z8320 - You won't decarboxylate 2-nitrosuccinate to nitroethane. Only the carboxylic group to which the nitro group is alpha can be removed readily.
|
I thought that might be the case, but I figured it wasn't too ridiculous considering that an amino acid can be decarboxylated. The easy solution
here would be to use an alkyl halide instead of an alpha-halo acid. So pass dry HCl or HBr into boiling methanol, and you'll be set. Condense with
dry ice if you're using HCl. This way you can alkylate and you don't have to worry about decarboxylating anything.
If you were both a chemical and a mechanical engineer, you might have a case about being qualified to build industrial chemical equipment. In lieu of
that, this is an article with some words on titanium's resistance to nitric acid corrosion. http://www.azom.com/details.asp?ArticleID=1240.
I've attached a paper on the vapor phase nitration of saturated hydrocarbons. I suspect if you read this, you'll change your mind about certain
aspects of your reaction, for instance, your temperature will not result in rearrangements to lower nitroalkanes; The only nitrated products you'll
get will contain three carbons.
Attachment: Vapor Phase Nitration.pdf (591kB)
This file has been downloaded 1809 times
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Sometimes hints are found in the original literature, and personally I'd consult that for Such A Project, but I'm a wet blanket with no sense of
adventure.
Mr. H. must not have written any articles or patents worth reading, since this thread, and Ullmann's "detailing the industrial route" doesn't mention
them.
|
|
|
Z8320
Harmless

Posts: 7
Registered: 13-12-2009
Member Is Offline
Mood: No Mood
|
|
Another thought I had was that you could take ethyl methyl ketone and form the corresponding alpha,alpha' dinitroketone with an alkyl nitrite or
whatever other agent gives the same result; I'd be willing to bet a less dangerous and illicit alternative could be found. Then you could alpha
halogenate and cleave the carbonyl with base in a manner analogous to the haloform reaction. You then decarboxylate the resulting alpha nitro
propionic acid to nitroethane. If that works you could adapt it to any methyl ketone.
The main concern I have is whether or not alpha halogenation works in this situation; you might get halogenation on the higher alkyl side just like
you do in the haloform reaction. Someone with MEK, access to alkyl nitrates, free time, and bleach should try this and see what can be isolated.
[Edited on 4/15/2010 by Z8320]
[Edited on 4/15/2010 by Z8320]
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
I'm a chemical engineer, among other things. With that sort of proposed pilot-plant scale process, I would be most concerned about the proper
pressure-vessel design of the piping and reaction vessels, to safely withstand the vapor pressures of the reactants at the desired temperatures, and
also to ensure that there are no leaks of hydrocarbon vapors that could result in an explosive mixture with air. The design would have to be in
HNO3-resistant materials, e.g. Ti and vessels lined with glass, with extra thickness to be allowed for any likely loss of material from the inside
surfaces due to corrosion. That reaction vessel, if at 420ºC, would have to be at a temperature which is safe for containing nitroalkanes without
exploding due to autoxidation.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Z8320  | | ... I suspect if you read this, you'll change your mind about certain aspects of your reaction, for instance, your temperature will not result in
rearrangements to lower nitroalkanes; The only nitrated products you'll get will contain three carbons. |
Actually, right near the start of the paper is the statement | Quote: | | As a result the Commercial Solvents Corporation established an efficient industrial process for the production of nitromethane, nitroethane,
1-nitropropane, and 2-nitropropane by the reaction of nitric acid and propane at temperatures above 400' C. |
and I've read elsewhere that nitration of propane in the range 420-450 C gives considerable amounts (10-25% each) of MeNO2 and EtNO2.
And Melgar, I'd not heat the tank of propane, as it's already under pressure. Instead place an inline heater between the propane tank
and the HNO3 tank to heat the propane gas to the desired temperature. Use a valve on the propane tank to control flow, it'll be much more responsive
than heating the tank.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Yep, and the verdict is that titanium is a superb material for containing nitric acid at virtually all temperatures and pressures. In case you were
warning me about the runaway reaction, I was aware of it. My plan was to dilute the nitric acid to like 90% or so, since that runaway reaction seems
to only happen at concentrations of 98%+. Since titanium is expensive, I will probably use 304L stainless steel for the parts of the reactor at low
temperatures.
| Quote: | | I've attached a paper on the vapor phase nitration of saturated hydrocarbons. I suspect if you read this, you'll change your mind about certain
aspects of your reaction, for instance, your temperature will not result in rearrangements to lower nitroalkanes; The only nitrated products you'll
get will contain three carbons. |
Thank you, this was very helpful, as it gave a very good description of how changing different parameters effects output. Although the reaction does
seem like it'd work at my initial values, after reading this, my plan is to lower the pressure to 50 psi or so, raise the temperature to the point
where I'd use molten aluminum to regulate it, (melting point 660 C, and no, it doesn't dissolve titanium), and then use heavy-duty glass for the HNO3
and collection vessels. At 50 psi or less, I'd feel perfectly fine about using vessels rated at 80-100 psi. Plus, I already have a pressure gauge so
I can closely monitor the pressure, and if I really wanted to make it safe I could install a burst-type valve rated at 75 psi or so, leading into a
bucket of water. Also, I'm thinking I shouldn't have it loop around three times, since that'd complicate my design too much.
[Edited on 4/16/10 by Melgar]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by not_important  | | And Melgar, I'd not heat the tank of propane, as it's already under pressure. Instead place an inline heater between the propane tank
and the HNO3 tank to heat the propane gas to the desired temperature. Use a valve on the propane tank to control flow, it'll be much more responsive
than heating the tank. |
If I had to get the pressure up to 150 psi, then I'd have to heat the propane tank in order to increase the vapor pressure to that level. See http://www.engineeringtoolbox.com/propane-vapor-pressure-d_1... . If I ran it at 50 psi instead, I suppose I could put the propane tank in ice
water to ensure that it won't go above that pressure. Controlling it via the valve would be easier, but probably subject to more fluctuation unless I
used a regulator. Still, this has actually all been good news since now I know I can run it at lower pressures and thus less inherent danger.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
You really don't need pressure for small scale setups; I've seen vapour phase nitration of butanes run at 2 bar.
Rule 12 of the reference given earlier:
| Quote: | | Elevated pressures increase the reaction rate and the difficulty of temperature control without greatly increasing the yields; however, the effect of
increased pressure is more important as the hydrocarbon series is ascended. In extreme pressures, nitration, presumably a second-order reaction, is so
rapid that proper temperature control is very difficult |
Rule 13 discusses the undesirable effect of catalysts and the possible need to pretreat the reactor lining.
Also not that contact times are critical and need good control, they generally run a fraction of a second and typically are followed by quick cooling.
Maintaining a large excess of the hydrocarbon is useful for both good yields and reducing the possibility of runaway reaction; dilution with N2 helps
there as well.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Blah, accidentally reloaded this page and lost my nice long response.
I'm finding gas grill propane regulators online for cheap, some of which can regulate from 0 to 30 psi. I'm thinking I'll use one of these.
Also, you can dilute with several different neutral chemicals. I plan to dilute the HNO3 with water, in order to not have to make another inlet and
make the HNO3 safer.
I'm a little concerned about the possible catalytic effect of titanium on the reaction. I've found a lot of papers describing titanium dioxide's
photocatalytic oxidation ability, but none describing it as an oxidation catalyst in the absence of light. I may have to gold-plate the inside of the
tube if it comes to that.
So now I'm thinking, single-pass reaction at low pressure in a titanium tube, with temperature regulated by molten aluminum. The titanium tube is, I
believe, 70 cm long and 7mm in diameter. Fittings will probably all be stainless steel compression fittings.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Damn, just had an idea. Would have been nice if I'd thought of it before I ordered all that crap. Anyway, alanine and beta-alanine are both
available at health food stores, as free amino acids. How hard would it be to oxidize the NH2 to NO2, then decarboxylate the thing? Theoretically,
alanine would give nitromethane and beta-alanine would give nitroethane. Could it work?
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
I've seen a method that bubbles the gaseous hydrocarbon through hot nitric acid, then into the bottom of a nitrate molten salt bath for
reaction....Where is that damn patent....
http://pubs.acs.org/doi/abs/10.1021/i260018a011
http://v3.espacenet.com/publicationDetails/originalDocument?...
[Edited on 4-19-2010 by Eclectic]
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
Quote: Originally posted by Melgar  | | How hard would it be to oxidize the NH2 to NO2, then decarboxylate the thing? Theoretically, alanine would give nitromethane and beta-alanine would
give nitroethane. Could it work? |
Acetonitrile won't be too hard, but you'd probably have a hard time getting the hypofluorous acid at the health food store...
sparky (~_~)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
You don't need HOF, you can easily make it by bubbling fluorine gas through aqueous acetonitrile.
Unfortunately, while there are several standard methods for oxidising amines to the corresponding nitro compounds, the referencee sparkgap gave seems
to be the only one that gives decent yields if any non-zero yield at all..
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Quote: Originally posted by Melgar  | | Damn, just had an idea. Would have been nice if I'd thought of it before I ordered all that crap. Anyway, alanine and beta-alanine are both
available at health food stores, as free amino acids. How hard would it be to oxidize the NH2 to NO2, then decarboxylate the thing? Theoretically,
alanine would give nitromethane and beta-alanine would give nitroethane. Could it work? |
You would more then likely be better off to perform the decarboxylation first using a high boiling point ketone as a catalyst and heat to yeild EtNH2
or MeNH2 followed by the oxidation of the amine with a mixture of oxone and acetone. There are references provided at The Vespiary for the process
using the oxone.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Yeah, I found that one too, then immediately ruled it out when I saw it involved gaseous fluoride. Still, I know I read once that you can just
combine amino acids with sodium hydroxide to get the sodium salt, then just heat until they pyrolyze. Supposedly it doesn't give such good yields for
more complicated amino acids like tyrosine, but for simpler ones like alanine or beta-alanine, yields are better. Anyway, I got some beta-alanine
from the health food store, then mixed some in an aqueous solution of NaOH. Then I boiled all the liquid
|
|
|
| Pages:
1
2
3
..
6 |