| Pages:
1
2
3 |
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: |
Place about 250mg of the compound in the tube and pack
lightly with the glass rod. (avoid holding the tube in your hands for this step). |
A wooden dowel would be safer than a glass rod but even holding a dowel while tamping HMTD seems pretty iffy.
Mounting it on a drill-press with a protective screen between you and the detonator should be the way to go!
|
|
|
Blasty
Hazard to Others
  
Posts: 107
Registered: 25-7-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Liedenfrost  | Quote: Originally posted by Blasty  |
"Detonating Composition To Replace Fulminate Of Mercury.— This composition is the invention of Herr J. Fuhrer of Vienna, and it is intended for use
in place of fulminate of mercury for producing the initial detonation necessary for firing a charge of high explosive. It is very much safer to handle
than fulminate of mercury and the inventor claims it will satisfactorily detonate the high explosives. If so, the gain will be very great. One of the
great dangers in handling high explosives has been the sensitiveness of the fulminate fuze or detonator, and no satisfactory detonator not containing
fulminate of mercury has (unless this proves to be the long sought article) been obtained. The materials used are copper, ammonium nitrate,
potassium nitrate, sulphur and aluminum. One formula specifies 30 to 40 parts of copper-ammonium nitrate, 42 to 25 parts of nitrate of potassium, 10
to 7 parts of sulphur, and 18 to 28 parts of metallic aluminum. The date of acceptance of the British patent (No. 20,755) is December 31,
1901." (Proceedings of the United States Naval Institute, Volume XXVIII, Part 1, page 145.)
|
Can you say damn sensitive Tetramminecopper(II) nitrate, often lazily and exasperately acronymised as TACN?
http://www.sciencemadness.org/talk/viewthread.php?tid=1778&a...
http://mihailru.freeservers.com/shopping_page.html
http://www.ab.ust.hk/hseo/sftywise/199302/page3.htm
Bad idea, even before we come upon the Al and nitrate dangers. |
I haven't tinkered with that compound (yet) but in this very forum I have read several differing opinions about how sensitive (or even if useful at
all) it is:
http://www.sciencemadness.org/talk/viewthread.php?tid=2187
|
|
|
Blasty
Hazard to Others
  
Posts: 107
Registered: 25-7-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by gregxy  | I doubt that these compositions work.
Here is a simple way to test primaries,
Wrap a 1.5" square piece of Al foil around a 0.25" glass rod to form a tube.
Crimp over the bottom end.
Place about 250mg of the compound in the tube and pack
lightly with the glass rod. (avoid holding the tube in your hands for this step).
Insert a fuse and close the top.
Tape the little cracker on a block of pine, light the fuse and
get away.
Most "flash" mixtures will simply burn since the confinement
is not strong enough.
A primary like fulminate or HMTD will explode and the explosion will cut a deep grove (about 1" deep) into the
wood block.
|
Some of these mixtures are meant to work under strong confinement only, otherwise they either do not explode at all or not with sufficient force to be
useful. Such a method of testing would not work for them. Stronger containers would need to be used.
|
|
|
Blasty
Hazard to Others
  
Posts: 107
Registered: 25-7-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by argyrium  |
Quote: Originally posted by Blasty "Care to elaborate on which of these precipitated mixtures you've found particularly dangerous to deal with?"
Notes unavailable, another lifetime. Same procedure had been done once before on a smaller scale.
A solution of K2CrO4 dripped into a solution of Ba(NO3)2 with fine B suspended therein. Don't recall concentrations or T other than it was warm
(preheated), w/ strong mechanical (overhead) stirring. Sometime after completion of rxn the 600mL beaker "exploded". The noise was like a very loud
'click' and nothing like the sound of glass that just fails thermally. The beaker broke into many small pieces, some being projected across a wide
bench. Addition funnel did not break.
No light or flash was noticed. Stirrer had not touched the glass and rate was a nice vortex to a near the bottom of the vessel.
Nasty mess to clean-up and gave someone quite a scare.
|
Messy stuff! Now care to elaborate on any safer ones you might have tinkered with in "another lifetime"?
|
|
|
Blasty
Hazard to Others
  
Posts: 107
Registered: 25-7-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by hissingnoise  | | Quote: |
Place about 250mg of the compound in the tube and pack
lightly with the glass rod. (avoid holding the tube in your hands for this step). |
A wooden dowel would be safer than a glass rod but even holding a dowel while tamping HMTD seems pretty iffy.
Mounting it on a drill-press with a protective screen between you and the detonator should be the way to go!
|
I once had an aluminum tube with some HMTD go off while pressing it in such a set up. I had placed lead ingots surrounding the tube, so they absorbed
most of the shock and shrapnel. My ears were ringing for a couple of days, though. The wooden dowel "vaporized".
[Edited on 22-2-2010 by Blasty]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: |
I once had an aluminum tube with some HMTD go off while pressing it in such a set up. |
I can see how that would make one look for safer primaries.
But HMTD detonating during pressing into Al should be a very rare occurrence.
Do you have any idea why it happened?
I once pressed incompletely (smelling as fishy as the hexamine used to prepare it) washed HMTD in a Al tube over a base-charge of RDX while holding
tube and dowel in my hand (hope this doesn't upset argyrium?).
My senses were heightened while I did it but no digits or organs were damaged!
[Edited on 22-2-2010 by hissingnoise]
|
|
|
gregxy
Hazard to Others
  
Posts: 421
Registered: 26-5-2006
Member Is Offline
Mood: No Mood
|
|
The point of my experiment is that a real primary does not
need confinement. It will also show what a tiny amount
it takes to remove your fingers.
Black powder will go boom if you put it in a steel pipe simply
because the presssure builds up and ruptures the pipe.
But the peak pressure is just a few hundred PSI. A detonating explosive will generate 100X more pressure.
You can also try taping an M80 to a wood 4X4. What
happens to the wood? Not much.
Quote: Originally posted by Blasty  |
Some of these mixtures are meant to work under strong confinement only, otherwise they either do not explode at all or not with sufficient force to be
useful. Such a method of testing would not work for them. Stronger containers would need to be used. |
[Edited on 3-1-2013 by Polverone]
|
|
|
Blasty
Hazard to Others
  
Posts: 107
Registered: 25-7-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by hissingnoise  | | Quote: |
I once had an aluminum tube with some HMTD go off while pressing it in such a set up. |
I can see how that would make one look for safer primaries.
But HMTD detonating during pressing into Al should be a very rare occurrence.
Do you have any idea why it happened?
I once pressed incompletely (smelling as fishy as the hexamine used to prepare it) washed HMTD in a Al tube over a base-charge of RDX while holding
tube and dowel in my hand (hope this doesn't upset argyrium?).
My senses were heightened while I did it but no digits or organs were damaged!
[Edited on 22-2-2010 by hissingnoise] |
I suspect it was because the bottom of the tube had not been as flattened as I thought it was. You see, I was trying to make an improvised "capsule",
rather than just shut one of the ends of the tube by drawing the ends together. I tried flattening the bottom of the imrovised "capsule" by inserting
an iron rod in the open end and hammering over an anvil, but I guess some rough and/or uneven surfaces remained. When I pressed the HMTD some friction
with the rough/uneven surfaces at the bottom of the tube must have happened and BOOOOM!!!
|
|
|
Blasty
Hazard to Others
  
Posts: 107
Registered: 25-7-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by gregxy  | The point of my experiment is that a real primary does not
need confinement. It will also show what a tiny amount
it takes to remove your fingers.
Black powder will go boom if you put it in a steel pipe simply
because the presssure builds up and ruptures the pipe.
But the peak pressure is just a few hundred PSI. A detonating explosive will generate 100X more pressure.
You can also try taping an M80 to a wood 4X4. What
happens to the wood? Not much.
Quote: Originally posted by Blasty  |
Some of these mixtures are meant to work under strong confinement only, otherwise they either do not explode at all or not with sufficient force to be
useful. Such a method of testing would not work for them. Stronger containers would need to be used. |
|
These mixtures however are claimed to explode so violently when confined that they allegedly can initiate at least some types of high explosives. The
claims of their inventors are not merely those of a firecracker-like explosion (Castellanos' patent from the 1870s even contrasts the much stronger
concussion of his "percussive petards" with that of black powder firecracker-like charges, which he points out could not reliably detonate dynamites.)
[Edited on 3-1-2013 by Polverone]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Pressing HMTD into a crimped Al tube could be dodgy too.
Pressing onto a base-charge like RDX or PETN should generate less friction but the consequences if it did go off would be more considerable than using
HMTD alone.
A small tissue plug in the crimped end of the tube might be another idea?
|
|
|
Lambda
National Hazard
   
Posts: 566
Registered: 15-4-2005
Location: Netherlands
Member Is Offline
Mood: Euforic Online
|
|
HMTD is exceptionally hazardous to press, it explodes, if pressed too hard. Too hard is really not hard at all, no Machines are allowed! Pressing by
Hand (with proper safety precautions) is OK. Also, the cap should never be made of Metals other than Aluminum, since HMTD is totally incompatible with
most Metals. If the Cap does not explode during manufacture, then it will deteriorate in a few hours/days due to the catalytic action of the Metal.
Copper and it's Alloys are particularly efficient in this respect.
Pink Floyd - Comfortably Numb - Pulse 1994 (HD Live):
http://www.youtube.com/watch?v=iJZYG5qwHHI
Lambda
|
|
|
dts
Harmless

Posts: 2
Registered: 27-7-2007
Member Is Offline
Mood: No Mood
|
|
Some old English patents appertaining to mixtures intended to substitute primary explosives
The described mixtures are interesting but largely too sensitive ( because of the chlorate ) to be useful. Does anyone know if replacing a proportion
of the chlorate with perchlorate :- a makes them safer and b retains their explosive properties?
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Chlorate mixtures with red P or sulphur are certainly too sensitive to friction and shock to be trusted but in any case, replacing chlorate with the
per-salt, if you can, is always a good idea.
|
|
|
dts
Harmless

Posts: 2
Registered: 27-7-2007
Member Is Offline
Mood: No Mood
|
|
chlorate perchlorate ratio in mixtures
My above post was perhaps not clear enough so ...
One of the mixtures described in Roscos post above is for trinitronaphthalene ( unspecified isomer ) and potassium chlorate. My question was how much
chlorate can be replaced with perchlorate to leave the mixture much safer but still retain the interesting explosive properties ( apparently able to
function as a primary ). ?
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
It's possible that replacing all the chlorate with perchlorate would make the mixture insensitive to the spit of a fuse.
A small quantity of perchlorate flash-powder pressed lightly on top should correct this.
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
IMO there is no "safe" or "safer" w/ Energetic Peroxides. Use powdered Al always as a dry lubricant when materials that are friction sensitive are
pressed. Avoid glass or seemingly smooth surfaces, as these actually contact the material harder on a microscopic level, etc. The pores of wood allow
Al or graphite to fill with a dry lube.
When viewing a film of a loading machine, one thing I noticed right away was the thing went very slowly. So slowly in fact that at first it appeared
to not be functioning.
Sometime back there was a thread somewhere about accidents and HMTD was right up there with suspicious consistency.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Thanks for the heads up on glass quicksilver - I have been considering glass tubes for HMTD.
But when I hear of HMTD accidents I'm inclined to assume some kind of careless handling, energetic pressing, incompatible metal or
insufficiently-washed product.
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
It's (HMTD) the only energetic peroxide that the US Bureau of Mines (USBoM) ever considered and tested. At that time (1912 + or -) plastic and
polymers were really unavailable but they did have bake-a-Lite and that was what they used for testing initiation. They found that from that
standpoint HMTD was quite respectable in initiation however the shelf life was much lower than Hg Fulminate. And that was exactly what they were
attempting to try and move from. this was the first time that azides were examined commercially & by a gov't testing agency. Scores of material
are available from the USBoM (now defunct) but reprinted in both white papers and a book specifically dealing with the subject. Detonators for High
Explosives; Hall, Howell, & Taylor - ISBN 1-4276-1460-1. The book is where most of the material is re-printed. I searched for quite a long time to
get the originals in their entire length.
And outstanding experiment was making dinitrohexamine as one were to make RDX but instead, use that to make HMTD. The resultant appeared to have
perhaps one of the most interesting properties. It appeared stronger than HMTD yet the yield was somewhat less! The material appeared to be longer
lasting (shelf life) and popped when exposed to flame instead of burn on a sample of tens of milligrams level. It appears granular instead of somewhat
"soft" and in all likelihood is more sensitive if mishandled.
Another very interesting paper was written on the development of a "sealant" for fuse caps. This is the first time I had read of the commercial use of
nitro-cellulose lacquer. The material was made to instantly transmit the spit of a flame and drive a very hot deflagration into the cap. The material
was made thick (25% NC) mixed with coal dust and K Chlorate. The transmission of flame becomes a "pop" but it is also waterproof. This material was
patented by the Austin Powder Company. Not only did it hold up to time but the material because it was so high in NC was pliable even when aged. This
made for consistent water proofing and had been used in Hg Fulminate fuse caps for many years. In cold climates the seal was sufficient to allow the
caps to last longer than the previous standard which was very high sulfur ratio BP seal (this was also warmed prior to mixing forming a tar-like mass)
pressed with the same pressure as the rest of the cap.
The BP seal often crumbled over time and was rather thin so as to maintain immediate ignition.
We all know that sulfur was used for plugs in both types of caps, as was tar. This continued despite better sealants because the tooling for warming
and delivery into caps made alteration of this, cost prohibitive.
[Edited on 2-3-2010 by quicksilver]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Naoum thought highly enough of HMTD to suggest its use in explosive rivets in a 1938 patent.
Presumably the explosive wouldn't be safe if pressed directly into the bare rivet but into some kind of protective capsule which could be placed in
the rivet's shank.
The patent however, made no mention of protective containment!
Whether the rivets were manufactured in number is doubtful too, considering HMTD's thermal stability.
Once loaded though, caps containing HMTD should be safe and fairly stable as long as they're properly stored and handled.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Not all that glitters is gold, and that often holds true for what may seem at first to be sparkling bright ideas 
[Edited on 3-3-2010 by Rosco Bodine]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
A single mishap will put me firmly in Blasty's camp.
If I do come a cropper I hope not to see any glistering in the hands area.
FLW?
No, but seriously, I've heard of HMTD going off while being pressed or from being left in sunlight (or was that AP?) but not from normal handling of
finished detonators.
[Edited on 3-3-2010 by hissingnoise]
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Actually safety would be a damn good thing to start a thread about. There are people who really don't understand static's impact on energetic
materials and often use the term "unstable" when the materiel was poorly synthesized or handled / stored inappropriately. That's one thing I despise
about AP; it's simplicity of mfg.
I once read a poster that actually didn't understand static ignition in relation to primaries. Again, only my opinion but I believe that static
ignition is responsible for some people calling some materials "unstable". Properly synthesized and maintained very few things are actually unstable.
If a material requires neutral ph, low temp, dry or moist environment, no UV - that does not make that material unstable. Way too many people want to
race through a synthesis so they have "run-a-ways" (when lucky) or the result is a shit-blob of wasted chemicals. When unlucky they feel that time
distorted sickly weak feeling and can't seem to hear or catch their breath as they feel nauseated with fear as they look at their extremities &
can't seem to focus on what just occurred.....
There used to be a quite lengthy thread on (UseNet) Rec.Pyrotechnics of "accidents". There are VERY few accidents with energetics: just as with
firearms, there is negligence. That's why I've not used the term accidental discharge -the damn thing was a negligent discharge. An accident is
actually tough to find if you think about it. However it could just be a question of semantics. Many people say "unstable" when what they really mean
is "sensitive" or demanding or certain protocols.... 
One serious problem is that few people READ (study) a hobby anymore. They want to race when they should be learning how to crawl without skinning
their knees.
[Edited on 3-3-2010 by quicksilver]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Over the past 10 years I've probably pressed well over a hundered blasting caps, never had a premature detonation while pressing the primary. Mostly
used Ag2C2*AgNO3, Pb(N3)2 and AgN3 for very small detonators, but ALWAYS in combination with a basecharge that is very sensitive to initiation, like
PETN, MHN or RDX! Never liked pressing the primary over the basecharge though, so I usually pressed the basecharge and primary seperately. The primary
was pressed in a somewhat smaller diameter tubing, that fits precisely in a somewhat larger tubing, in which the basecharge was pressed. This allows
you to press (and store) the primary (20-100 mg), attach fuse mechanism, etc without any risk of prematurely detonating the basecharge. To decrease
the amount of primary needed I also pressed about 100 mg of the basecharge by hand (~1 g/cm3) on top of the lever-pressed or plasticized basecharge,
(>1.6 g/cm3) for easy initiation...
Usually used STRONG plastic tubing as the casing (you dont want it to flex with the primary inside!!!!) and a large length of it. Adding some length
to your detonator casing not only makes pressing of the basecharge easier but also provides sufficient distance between your fingertips and the
basecharge when handling the finished detonator. (When plastic is used)
[Edited on 4-3-2010 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
With regards to fulminating mixtures containing KClO3, I have wondered about
the possible use of manganese ferricyanide as a combination fuel and catalyst,
as the MnO2 byproduct is a known catalyst for the heat driven decomposition and release of oxygen by KClO3. Reportedly the addition of potassium
ferricyanide
to a solution of a soluble manganese salt results in the precipitation of the
manganese ferricyanide Mn3[Fe(CN)6]3 as a brown substance insoluble in HCl,
also insoluble in NH4OH. I almost hesitate to speculate about such materials possible usefulness with KClO3 because there is an inherent danger
associated with KClO3 mixtures which have a reputation for instability which is unpredictable,
and some of the KClO3 containing mixtures have even reportedly undergone spontaneous ignition or detonation, without the helpfulness in that regard
which
would be supplied by any catalyst deliberately added. Therefore I would include a caveat with my suggestion about the possible usefulness of
manganese ferricyanide as a catalyst, that such mixtures conceivably could experience storage instability, including the risk of spontaneous ignition
or spontaneous detonation. Any experiments with such mixtures should involve small quantitites
and provisions for safety that allow for such a variable as that unknown control
introduced by the uncertainty of whether the energetic material will initiate
from a deliberately applied stimulus, or equally well at any other time it pleases.
It is precisely that uncertainty about such mixtures which causes my lack of enthusiasm for these types of pyrotechnic mixtures, and encourages my
interest in chemical compounds
which tend to be more predictable.
[Edited on 14-3-2010 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
A related idea which I have been considering as a possibly worthwhile experiment regarding the energetic combination of ferricyanide and chlorate,
involves a possible means of compounding the materials as a clathrate rather than as a physical mixture.
Referencing the attached file, Lead ferricyanide exists, and forms also a basic salt, and a double with lead nitrate.
Lead nitrate is also known to form complex multiple salts including combinations with lead chlorate and basic lead picrate, which may also contain
organic salts of lead such as lead acetate or formate or glycinate ......therefore it would seem possible that lead ferricyanide may be included in
that scheme as well. If my postulate hold true, then it should be possible to form a basic lead picrate - lead nitrate - lead chlorate - lead
ferricyanide multiple salt or clathrate with
the idea being of course to achieve an intimate mixture at the molecular level which brings together the chlorate and ferricyanide in an energetic
matrix of basic lead picrate - lead nitrate. It is probable that such a multiple salt would have energetic properties. And it is also possible that
such a composition if it exists is novel and unreported.
Reportedly 3 grams of Potasssium Ferricyanide dissolved in 7 ml H2O when mixed with 3 grams of Lead Nitrate dissolved in 8 ml of H2O will after a few
minutes yield more than a gram
of crystals of Lead Ferricyanide. These crystals could be obtained separately in advance by this method if necessary for use as a precursor. Only
part of the ferricyanide is precipitated as Lead Ferricyanide and the residual solution
contains a double salt of Lead and Potassium.
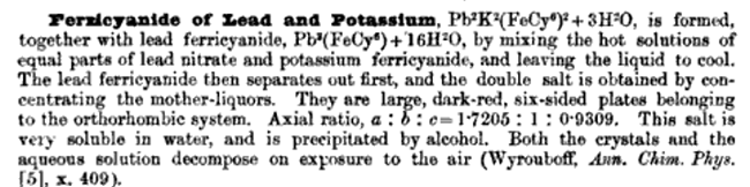
 Also of interest is something which I have never seen before from an old text
circa 1891 reporting that Potassium Ferricyanide itself will detonate on heating with Ammonium Nitrate. Now that is an obscure and interesting bit of
information. Also of interest is something which I have never seen before from an old text
circa 1891 reporting that Potassium Ferricyanide itself will detonate on heating with Ammonium Nitrate. Now that is an obscure and interesting bit of
information.
Attached is a separate file which indicates that extreme caution should be observed for the inhalation hazard associated with some reactions involving
ferricyanides because of the posible release of cyanide gas which can occur from these reactions. Also may be encountered a danger from the formation
of cyanide salts.
The nitroferricyanides (nitroprussides) are also of potential interest.
Attachment: lead ferricyanide related.pdf (165kB)
This file has been downloaded 788 times
Attachment: ferricyanide reaction hazard warning.pdf (211kB)
This file has been downloaded 923 times
[Edited on 18-3-2010 by Rosco Bodine]
Attachment: Nitroprussides.pdf (259kB)
This file has been downloaded 1973 times
|
|
|
| Pages:
1
2
3 |