| Pages:
1
..
32
33
34
35
36
..
48 |
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Swede,
You have got yourself some lovely Platinum coated Ti substrates for Lead Dioxide Anode's there...................
Doing a calculation from the 3 Micron thick coating of Pt I was amazed to find out that the total Pt per square Meter is indeed 64 grams per square
meter. (You quote 50 but it's around the same). That's 64 X 50 (Dollars per gram, roughly) = $3200 of Pt on a square meter 
Do you know if the Pt on the Anode's is actually electroplated onto them or put on with a chemical decomposition method. (bakeing I guess)? Just
wondering for the record.
EDIT:
There is a very dodgy dude selling dodgy materials on ebay here though I must admit 4 + grams of ChloroPlantinic acid would be great....... O stop Dann2
I think I will stick to the less dodgy black molars   
Dann2 (going off to the morgue)

[Edited on 19-1-2010 by dann2]
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Hahaha! That is awesome! I don't know jack about Pt dental implements, but I thought gold was more commonly used. At 4.2 grams for that molar, we
are looking at 0.135 troy ounces, and at $1600 per troy ounce, you're looking at $216 worth of Pt.
It sucks that Pt is so expensive. Why can't it be more like silver? We'd never have to worry about a good perchlorate anode again.
I'll gamble that it will sell for about $180 given it's state... perhaps stolen from a dead person.
For small quantities of platinum, I'll bet there are 1/10 and 1/4 ounce bullion coins out there of 0.999 Pt, nice and pure.
The use of a Pt mesh to act as a substrate for LD is an interesting one. The worst case is that all the LD falls off, and you are left with... a
valuable Pt anode. You really don't lose anything except the work. If it DOES work, then you have zero Pt loss so long as the LD adheres and
functions properly.
[Edited on 20-1-2010 by Swede]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Thoughts on Cathodes
Hello,
Perhaps we need a new thread!
I set up a pH controlled Na Chlorate cell today using a Graphite Anode. I intend to use different Cathodes in the cell to see if using big or small
Cathodes makes much of a difference when a cell is run without Cathodic reduction additives (like Chromate, Flouride etc).
I have three Cathodes I will be trying. Large Ti, small Ti and a large mild Steel one.
The large Ti and Steel Cathodes are shown in the picture. The shiney one is mild Steel. The other is Ti but it is more grey than the picture depicts.
I will be letting the cell stabalize for approx. two days untill acid additions are steady using small Ti Cathode. Then add large Ti Cathode for one
day, small one for next day, large one next day, small one next day, large steel one next day, small Ti next day, large Steel next day and then add
Dichromate next day (or something like that).
Samples of cell will be taken at the end of each day and titrated. Should show up any large advantages/disadvantages associated with using small/large
Cathodes.
The surface area of the two small flat Ti Cathodes (one each side of Anode) is 40 square cm, These two Cathodes have their backs covered with plastic.
The total surface area of the large Ti and mild Steel Cathodes are 714 square cm (2 * 357, counting back and front)
I am using a CC supply (homemade from computer power suppy) giving 4 Amps into cell.
Acid addition is with a syringe pump.
The cell I am using is the same as the one I used before for making Chlorate with Graphite with pH controll (way up the thread somewhere) and contains
approx. 2.2 litres.
@Swede
I would be inclined to agree that Sulphuric acid would definately be worth trying in a Perk. cell if it helps Anode erosion. Sulphates are not good
news with Chlorates so it would not be a good idea at all in a Chlorate cell (assuming is does any good in a Chlorate cell anyways and HCl is not a
problem in a Chlorate cell).
I am slowly but surely getting around to making a new LD Anode.
Cheers,
Dann2

|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Hi guys - I have worked a bit with the tubular Ti shank concept, and one of the real problems is the poor conductivity of the Ti tube. We tossed
about some ideas like filling the tube with lead or an alloy of lead, and using that to carry the bulk of the current. I bought some alloys and also
some 1/4" tin wire from Rotometals and attempted to fill a 1/2" OD Ti tube (flattened on one end for welding to an electrode) with tin so that the tin will carry the bulk
of the current rather than the Ti, a poor conductor.
It was painfully easy, AFTER heating the Ti to dull red:

The interior was scored for mechanical purchase:

And it was filled with pure tin using a simple propane torch:


The end of the tube was cleaned up, drilled and tapped 1/4" X 20, and is ready to accept a crimp terminal:

The whole idea was to create an electrode shank for production - solid and leak-proof mounting using a PVDF compression fitting, and an ability to
carry a current that puts the Ti tube skin to shame. An earlier attempt using a brass cap to make the connection:

So far, so good. Tin drills and taps well. I think this will work. 
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
@ dann2: I posted the former before seeing yours - I am really interested in seeing what sort of results you see. I know you are a fan of
appropriate cathode current density, and I hope you can gather data that will shed some light on this issue. I think the goals of most hobbyists are:
1) Preserve the integrity of the anode, be it Pt or LD
2) CE
I would happily give up some % of CE if it preserves the anode, especially when dealing with Pt. If there is a specific cathode CD or orientation, or
if there is an additive that helps, then it would be a real boon to the hobbyist.
I look forward to your resuts!

|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
Swede, if any salts appear at the top connection of that shank I will eat my boots!
I cranked up the cell approx. two days ago and am waiting for the cell to stabalize (to come to the point where a steady (known) rate of acid addition
keeps pH where we want it).
After one day with small Ti Cathodes I decided to put in the large Iron Cathode to age it a bit for the study proper. I am not 100% sure but it seems
that the pH actually started to drop too much when the Cathode went in and when I stopped acid additions the pH stayed at approx. 5.9.
Can anyone comment on what might be going on. If a large amount of reduction is taking place at the Cathode will this cause the cell pH to go lower or
stay lowish?
I will have to look up some cell chem. equations I guess.
On a slightly different note, since stainless steel has Chromium in it, perhaps it is not as bad a material for reducing wanted product back to
starting products as compared to mild steel. Did I read somewhere that it does form a (somewhat poor) anti reduction film. Not too sure.
@Swede
Did you come to any conclusions regarding end of cell run detection with your data gathering?
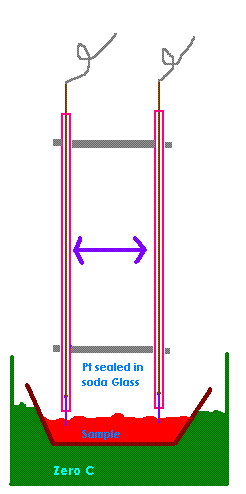
I was thinking of making something like the attached pic. A sample would be taken from the cell and measure at zero degrees (nothing special about
zero degrees it's just easy to do with ice). This would eliminate temperature problems. The distance between the probes would be fixed and could be
quite large is this was an advantage.
Pt is compatable with soda glass and you would only need a cm or two of Pt wire as it can be soldered to Copper like here.
Dann2
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
dann2: I like that concept that you have diagrammed, and I think it would work. I still have reams of data from the run, and I saw the exact same
phenomenon seen before - at CC; an intitially higher voltage requirement which dips within a short time, but then gradually climbs as chloride is
depleted. But then as we noted, it was strongly temperature-dependent, which really gums up the works as far as simply monitoring a cell's voltage
and current as compared to the initial conditions. If I was more of a mathematician, I'll bet I could come up with a crude but workable mathematical
model that relates voltage, current, temperature, to chloride, with a "constant" in there which will be derived via calibration, and which will vary
with cell geometry and materials, since each cell is wildly different.
I was thinking about this the other day when we were talking about conductivity or other simple measurements. I am tempted to mix up several small
samples, starting with, say, 15% KCl salt, and then introducing chlorate salt and reducing the KCl. Picture a series of about 5 small samples that
would be representative of a cell during production. A set of electrodes very similar to what you have pictured. Then measure... what do we measure?
Can it be as crude as resistance in ohms? And would this resistnace vary horribly with temperature, or could it successfully be ignored? Given the
very high (saturated, but varying species) salt content of the liquor, you would no doubt get an ohm reading depending upon the size and spacing of
your electrodes. Then, here comes pH to throw yet another variable.
If the temperature did not play a big role, it might yet be possible to get a reasonable idea of chloride based upon some very simple measurement, but
pH...
Anyway, I like the idea of removing temp from the equation by doing it at 0, as you suggest.
On the use of stainless as a cathode - the thought that the chromium content might reduce or eliminate unwanted reduction at the cathode is a good
one. But would the cathode be subject to erosion? Powered on, in theory, it would be cathodically protected and hopefully the liquor would remain
free of chromium, nickel, moly, and Fe.
[Edited on 31-1-2010 by Swede]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
The most useful measurements are measurements around the most useful operating point. That includes transient species like hypochlorite and all the
active stuff around an electrode (H2 and OH- at the cathode, H+ and Cl2 at the anode), things that can't be measured with two salts in water at zero
current.
Tim
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Tim, I agree, and my understanding and general P-chem and electrochemical knowledge is painfully weak... but I'm thinking of a very simple method to
determine rough end of run conditions. If it can get to within 1 to 2% chloride in terms of accuracy, I think that'd be successful, and since a
potassium cell (at least) only goes from 16% to perhaps 6% Cl-, it is a margin of error of 10% or more.
It'll be a simple ratio of chloride to chlorate, and I'm wondering if that ratio changes, is there a simplistic method to measure it using
conductivity? There are a few options now to measure or predict Cl- : you can titrate frequently or use expensive Hach Cl- titration strips; you can
create a mathematical model that is based upon current delivered and starting chloride; or, you can again create a mathematical model expressed as a
function of voltage, current, and temperature, and at a given temp, how the former two change in a given cell.
pH is a whole nuther ballgame. But these are starting thoughts.
@dann2: I re-read your cathode experiment, and am wondering - did you consider the differing environments when you do the cathode swap? CE isn't a
constant thing, it doesn't stay at 85% for the entire run, but varies. I'd guess CE is at its very best at probably the 1/5th point - the cell
chemistry is relatively mature, yet the Cl- is still high. If you swap cathodes through the run, each cathode is going to see a different Cl-
concentration. Still, it's infinitely better than anything I've tried, which is to simply stick with Ti in a size "that looks about right." The only
problem I ever had with Ti was the "banana" cathodes from the 95 C, zero chloride, near boil-out of my jammed electrode cell in the failed 2-cell
system. Those cathodes never did straighten out, despite heating with a propane torch, and plain old aging.
The question is minimization of reduction at the cathode. How to control it? We don't want Cl- attacking Pt. Choices are materials, additives,
current density, maybe temperature, maybe pH. pH control in a perc cell is problematic, and most simply don't bother.
Additives - different additives can wreck LD especially. I don't mind moderate toxicity like NaF but carcinogenics like chromium bug me personally.
Current density - high current density at the cathode is supposed to minimize reduction there, true? One method might be to try a run with a
ridiculously small Ti cathode. There will probably be a point where the protection vanishes and the Ti is eroded. A small-scale bench-top setup
could be run with a moderate MMO anode, very small Ti cathode, and progressively dial up amperage, giving each amperage a period of time, say 6 hours.
Then, weigh closely and/or visually inspect for erosion. Perhaps titrate for Cl- using a sensitive test. At some or perhaps multiple points, you'd
see a spike in Cl- concentration, visual Ti erosion, or both. That would be a limit for current density.
I'd expect low current + large cathode = reduction of ClO3 to Cl-, and higher current + small cathode = erosion. The sweet spot will be somewhere
towards the latter. I'd rather erode a $5 Ti cathode than a $150 Pt anode.
Enough babbling for now. I've got "T-Cell III" ready to go with some excellent improvements. It'll do another run with chlorate, then a perc run
using the tin-filled Ti shank welded to Pt. It has mechanical stirring using a PTFE rod + Ti paddles, and the previously mentioned HCl drip system.
I'll get some pics up. 
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
What I am doing with the Cathodes is not going to show a 'best' CD for Cathodes but (hopefully) show that it important to have a fairly sensible
Cathode size. Large 'stupidly big' Cathodes will seriously effect CE (I think).
See Ullman page 24 (and 9) at this link:
http://oxidizing.110mb.com/chlorate/further/ullman.pdf
The presence of Chromate film on Cathode effectively raises Cathode CD. We can do the same using a small Cathode and covering the backs (the part of
the Cathode away from the Anode) with plastic. A coaxial Cathode is going to have a very very low CD on it's back side (rude!). If a coxial Cathode
must be used it would be important to have it drilled (ie. a mesh) so that surface area will be smaller and also current will be higher on it's back
side as there will be a more direct line of site (as it were) to the Anode.
The different CE's I am going to get will be superimposed on the changing CE you obtain with changing Chloride but I am hopeing that I will get a good
indication of the effect of very large Cathode versus small (sensible) size.
Large or very large CD on a Cathode is not a problem and it will not erode it at all IMO.
Corrosion in the head space is a problem unless using Ti (or Lead or Graphite?). I am getting rusting of the mild Steel Cathode in the head space (its
very thin metal) so I am going to coat it with solder in the headspace area to protect it. I presume solder will not corrode?
Often wondered about a Lead Cathode. It would be easy to fabricate and cheap and non corroding.
Ti does not reduce Hypochlorite and Chlorate much I have read so a large Ti Cathode does not really matter (I think!), except for expence. (read this
in a Thesis somewhere or other).
Regarding the probes I guess it's going to be a case of suck it and see 
I am not to sure what exactly we are measuring and what is having the most effect at causing the cell voltage to rise. Less Chloride? More Chlorate?
I had not thought of pH. Perhaps it will not effect the reading much? Don't know myself.
I have splashed out on a one cm piece of Pt wire some time ago so I might try making the probes sometime and see. Small pieces of MMO might do instead
or even two pieces of Graphite.
Dann2
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Might niobium wire (reasonably cheap) form the basis of probes, or would it too passivate? Iridium is another metal that is not outrageous. Au?
Fine Gold wire is significantly cheaper than platinum. It could be mechanically twisted with Cu leads, and all except a tiny Au portion encased -
glass as mentioned, or possibly a polymer of some sort to keep the liquor off the Cu.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Ti CD: I have been corresponding with another hobbyist who apparently wrecked a Pt anode with excess current in an attempt to go from chlorate to
perchlorate. He used a CP Ti cathode, and declared he saw "Heat traces" on the Ti. I don't know if he is referring to the strap above the liquor,
where I too have seen heat discolor the Ti, or some other sort of traces or markings on the Ti working surface, but that could not be heat due to
immersion.
I am tempted to immerse several small wire samples in a full chlorate run to see if the wires suffer in any fashion.
[Edited on 1-2-2010 by Swede]
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Would a charge-meter work?
Charge is just the integral of current, and you're already sampling and recording current values. Pick an upper bound on current efficiency, and it
gives you a stop point. It won't run a liquor batch to completion, but it will keep the apparatus safe within its operating envelope. If you're
reusing the liquor, you'd want to characterize it between batches, but this doesn't require new gear. If you're not reusing it, the loss is a bit of
chloride feedstock and perhaps bath additives; this would trade off against the convenience of unattended operation and lower initial cost of
monitoring sensors.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
Regarding the charge meter IMO when you are using a CC supply it is very easy to know the amount of charge that has entered the cell. It's just time
by the CC. The charge meter would be good for the person with a Voltage supply where the current is inclined to vary as the cell progresses. But
either way you are back to guessing the CE in order to guess the end of run of cell.
Niobium (like Ti, W, Ta, Hf, Zr) is a Valve metal and will passivate when used as an Anode.
Gold will corrode but you may have 100's or 1000's of measurements done before it gives up. Iridium would work IMO. It's not as 'Noble' as Pt but the
electrodes are only going to spend minutes in the samples.
A few cm of Pt will only cost a few dollars. The piece I have is actually 3cm!!! long which is plenty for two probes. I will have a row of sample soon
taken from the Chlorate cell I have going at the moment and it would be easy to put them into ice water and 'measure' them all if I make the probes.
Exactly what Voltage (or current density) to put onto the probes is anyone guess. When I put approx. 3.0 Volts or more on the probes I will have the
worlds smallest Chlorate cell Since the cell is pH controlled all the samples
will have similar pH. Some of them will have been sitting there for days which may lead to 'wrong' readings? Since the cell is pH controlled all the samples
will have similar pH. Some of them will have been sitting there for days which may lead to 'wrong' readings?
Dann2
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by dann2  | | Regarding the charge meter IMO when you are using a CC supply it is very easy to know the amount of charge that has entered the cell. It's just time
by the CC. The charge meter would be good for the person with a Voltage supply where the current is inclined to vary as the cell progresses. But
either way you are back to guessing the CE in order to guess the end of run of cell. |
I'm suggesting that the
cell stops before it hits its end state. You're losing some yield on a per-batch basis, obviously. You're gaining unattended operation. This
trade-off might be just fine for some people.
This idea does require some amount of calibration for the cell and its operating profile. You'd want a first run whose duration is calculated
according to, say, 30% CE. When the cell stops, sample the contents and do a bit of quantitative analysis about what's in it. Start up again and
sample at, oh, 40% and 50%. You can extrapolate where end conditions are. Now back off 5-10% of where you'd stop the cell if you were continuously
monitoring. You're losing some per-batch yield, but it's not more that 15% or so. You'd be doing an extra batch for every five or six batches for
similar yield. If that extra work is worth it to avoid building a continuous monitor, then the trade-off is worth it.
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
You guys got me hooked on this. - An unbelievable amount of great ideas!
The computer PSU was fun to build and I got some really good ideas from the concepts.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Pt probes
Hello,
Good to see you hooked QuickSilver! I have lots and lots of Salt (it's very expensibe but you WILL want to buy it) for sale to start you off 
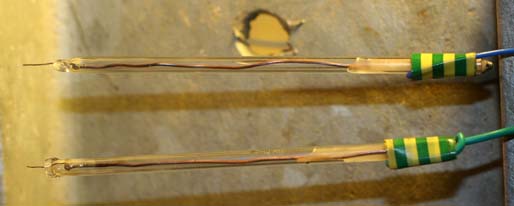
I constructed two Pt probes that may or may not be of use. The glass tubes are 5mm in diameter. I had only 4cm of Pt wire (approx. 0.5mm diameter).
Made one probe OK. The next one had the soldered connection to the Copper wire too close to the end of the Soda glass tube and also rubbing against
the side of the tube and the solder melted again to a high temperature for some time as I melted the tube shut. This seemed to dissolve the Pt wire as
it broke as soon as I moved the Copper wire (at the other end of tube) to bend it. Had to make it again and the bit of Pt sticking out of the end is
very short (half cm, as opposed to one cm on first probe). If making one of these keep the soldered joint in the middle of the tube, not lying against
the side of the glass tube where the solder will melt and damage the Pt (or use a longer piece of Pt so that the soldered joint is up the tube out of
the way).
I poured some candle wax into the tubes at the opposite end of the Pt to steady the Copper connecting wires.
What Voltage/current/waveform to put on the probes for to see a usable signal telling us of the cell stage?
Dann2
|
|
|
Lambda
National Hazard
   
Posts: 566
Registered: 15-4-2005
Location: Netherlands
Member Is Offline
Mood: Euforic Online
|
|
Quote: Originally posted by Swede  |
... It'll be a simple ratio of chloride to chlorate, and I'm wondering if that ratio changes, is there a simplistic method to measure it using
conductivity? ... |
Hi @Swede,
Wonderful work !, ... I love reading your posts, and seeing your progress !
The easiest way to measure Ions (Cl, ClO3, ClO4 etc.) is done in the same way as with a regular ION-SELECTIVE PH Meter, ... and yes, ... a PH
Electrode is an ION-SELECTIVE Electrode !
You can use a regular PH Meter for the readout, only now with different Electrodes (the principle stays the same though) that must be calibrated
according to the Ions that you intend to measure via an Ion Selective Electrode.
And this is what I am referring to:
Ion selective electrode:
http://en.wikipedia.org/wiki/Ion_selective_electrode
Just a few examples of what I am referring to (random examples):
Ion-Selectieve Electroden (Dutch):
http://www.rhombus.be/contents/nl/d151.html
Ion-Selectieve Electroden (Dutch) - Google Translation (Dutch to English):
http://translate.google.com/translate?js=y&prev=_t&h...
Technical Specifications for the Perchlorate Ion-Selective Electrode (ELIT 8061):
http://www.nico2000.net/analytical/perchlorate.htm
Ion Selective Electrodes : Ammonium (NH4+), Barium (Ba2+), Bromide (Br-), Cadmium (Cd2+), Calcium (Ca2+), Carbonate (CO32-), Chloride (Cl-),
Cupric (Cu2+), Cyanide (Cn-), Fluoride (F), Iodide (I-), Lead (Pb2+), Mercury (Hg2+), Nitrate (NO3-), Perchlorate (CIO4-), Potassium (K+), Silver
(Ag+), Sodium (Na+), Sulphide (S2-), Thiocyanate (SCN-), Water Hardness:
http://www.rmprocesscontrol.co.uk/Electrochemical-sensors/IS...
I hope that this info may be of assistance to your very interesting Chlorate/Perchlorate Project !
Lambda
|
|
|
Lambda
National Hazard
   
Posts: 566
Registered: 15-4-2005
Location: Netherlands
Member Is Offline
Mood: Euforic Online
|
|
Here you have a book, and two articles on Ion-Selective Electrodes:

Ion-Selective Electrodes for Biological Systems - By Christopher H. Fry & Stephen E.M. Langley (Harwood Academic Publishers - 2001) 157s -
Including Bookmarks.pdf
Ion-Selective Electrodes - By Mark A. Arnold & Mark E. Meyerhoff (Analytical Chemistry, Vol.58, No.5, pp20-48, April 1984).pdf
The Theory of Ion-Selective Electrodes - By Erno Pungor (PINSA-A, 64, pp53-65, January 1998).pdf
as
Ion.rar
iFile.it Download Link (8.69 MB):
http://ifile.it/u9ox3tz
No Password Required !
Enjoy !
Lambda
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
I obtained a paper entitled:
Simultaneous flow-injection measurement of hydroxide,
chloride, hypochlorite and chlorate in Chlor–alkali cell
effluents.
Some may be interested in taking a gauk.
It is in the ref. section under Wanted References and Needed Translations(6), post of 3 Feb 2010
Thanks to jokull.
I do not think its contents are of much use to us as the amounts of Chlorate are very low and the Chloride is measured using a conductivity cell but
the NaOH (main interference) is already known and thus can be eliminated.
They have an interesting way of measureing Chlorate with KI that is readable using a photo detector and a led.
Dann2
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
I secured a supply of carbon cutting rods and they were coated (as some of you know) with a layer of copper. This copper is no joke to take off. It's
perhaps a 1/2 mm in thickness and needed a a fine tip torch to cut a line in to peal off. But once they are off the graphite rode are well made. but
that copper has to come off and the rods need to be cleaned of copper left behind of one's product will be green as grass.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
@quicksilver
I found that the best way to peel of the Copper was to start at the top and put a small nick the Copper to lift a small part of it. Pincers were then
used to start off the peeling by rotating the rod and moving down the rod as the Copper peels off. You end up with a big long piece of Copper (all in
one piece) not unlike a spring.
Perhaps that is what you are doing.
If you controll cell pH (just add acid, no need for fancy pH meters etc) you should be able to get a few KG's of Chlorate made per rod. Without acid
you will be lucky to get one KG of Chlorate per few rods!
Attached is a read on conductivity of solutions.
Each ion has a specific condutcivity factor which may be useful. These devices seem to use an AC between two (blackened) Pt probes.
Cheap circuit to build one is here:
http://pubs.acs.org/doi/abs/10.1021/ed074p572
They can be bought on ebay too.
Dann2
Attachment: 12Chapter12.pdf (55kB)
This file has been downloaded 1080 times
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
@ dann2:
Thank you.
I had been reading this thread till I feel a bit up to speed on some of the more basic issues. Cutting Rods were said to have some iron: obviously
some more, some less; as all welding electrodes - how does one tell if you have a sweet brand or not? Or is this REALLY an issue? My agenda is to
re-crystallize, etc thus clean up my yield anyway.
I also read about cleaning up the Cu with HNO3 2% and linseed oil impregnation - these sound like a good thing. Have you done this also?
Tungsten is easily acquired via TIG electrodes...I have some - are they of any use in some electro-chemical format?
[Edited on 5-2-2010 by quicksilver]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
I attempted to coat W (Tungsten) with Tin Oxide (some time ago) using a similar procedure to Ti but could not get it to work.
It is very hard to etch with HCl too. I think a few W rods would make an everlasting Cathode for a small cell if you cannot get Ti.
There is a article on a cheap conductivity meter over in ref. section in the 'Wanted refs. and need translations (6)' thread, post of 5 Feb 2010, if
anyone is interested. I think the solution conductivity idea will definately work with a K cell because the Chlorate (or Perchlorate) is constantly
falling out of solution and the conductivity of the solution will be getting less and less (large signal) unlike a Sodium cell. The range of
conductivity is way off from what is usually measured with conductivity meters though. Far too much ions in solution. Most conductivity measurements
are performed on solutions with much less ions in them.
Dann2
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
I was reading back through this and other threads and had come across the concept that KCL does not dissolve well in cold water. I tried it and sure
enough, in water about 70-80C I was getting super saturated solutions. However I have a chlorate cell with spacing at 2.5" (MMO and Ti), with this I
have trouble with a super saturated solution. It will occasionally simply stop. I have check electrical issue so many times that I am confident that
it is not a PSU issue. The brine is saturated well over what I have tried in the past. When it's running at 4.5 -5v at 3A everything is fine (starting
at 12v via a PSU from a computer) but with most other voltage/current situations, it will not start. Is the highest level of saturation -something to
avoid?
I found a 5v 30A premium PSU at a surplus electronics store in town (was used by Raythion) for $30 but I don't see how I can overcome the heat issue
without a more sophisticated supply. I can also pick up a Lamda but I'm not sure if I want to gamble on it as they want $70 and that is a rack mount
high end 1970's power unit.
|
|
|
| Pages:
1
..
32
33
34
35
36
..
48 |