unome
Hazard to Others
  
Posts: 134
Registered: 17-10-2009
Member Is Offline
Mood: No Mood
|
|
Dimethocaine - using Panthenol?
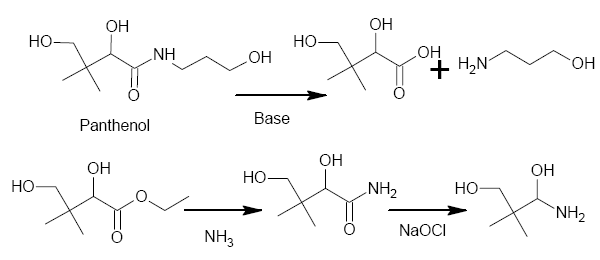
Panthenol has some structural similarity to Dimethocaine, especially since it cleaves, on basic hydrolysis, to the a-hydroxyacid and B-alaninol.
If one formed the ester from the acid, then the amide from the ester, a Hoffman would give an aminoalcohol which VERY closely resembles that of the
Dimethocaine side-chain (in fact, apart from that a-hydroxy, it would be identical.
Has anyone got any ideas how (or maybe, when) to remove that secondary alcohol?
PS The drawing doesn't take into account what would happen to the alcohols, primary or secondary, during the Hoffman Degradation. I think I would
check to see if that secondary alcohol can be selectively oxidized then run a modified Clemmensen reduction on it (both Zn & HCl are cheap &
readily available). Form an ester with the primary alcohol after the Clemmenson and prior to the Hoffman. I'll fix the drawing later, I'm out of time
now.
[Edited on 1-2-2010 by unome]
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Catalytic hydrogenation or reaction with something like LiAlH4 would reduce both -OHs to Hs, offering no differentiation. Dehydration of the -OHs
would be possible to form alkene double bonds only if there was also some energetically-favored structural rearrangement, but that looks unlikely, and
in any case would not be wanted. But oxidation with (di)chromate or permanganate, under certain conditions, would oxidize the primary alcohol -OH to a
carboxylic acid and the secondary alcohol to a ketone which in fact would be part of an amide group, which differentiation should provide some sort of
means of subsequently reducing the ketone; although there is possibly some danger that the primary amine group might also be oxidized.
The carboxylic acid group could be esterified with glacial acetic acid plus a small amount of H2SO4, or with acetic anhydride, to reduce its
reactivity. The problem would then become one of how to reduce the ketone part of the amide group to Hs while leaving the ester untouched. However,
esters also undergo catalytic reductive hydrogenation and with LiAlH4, as do ketones, so some other more selective method would have to be found.
[Edited on 1-2-10 by JohnWW]
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Hydrogenation is not a reasonable option. Stoichiometric lithium aluminum hydride, or cocktails of NaBH4 (say with H2SO4 or I2 to give borane) will
reduce the hemiaminal (last structure you have drawn. hydrolytically unstable functional group) to the amine, which it seems you're after. It will
not, however, reduce the primary alcohol to the alkane. I have no idea if that Hofmann rearrangement will work - my gut feeling is no, because the
reaction is run under aqueous conditions wherein the product is not stable. Thus, if you can even obtain the aldehyde product (without oxidizing it
during the reaction) you might consider reductive amination.
Dehydration of any of the alcohols drawn will not occur, as none possess alpha-hydrogens to affect the beta-elimination.
Look up the Lindgren oxidation for the hypochlorite oxidation, but this is under acidic conditions.
[Edited on 1-2-2010 by Arrhenius]
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Wouldn't acid hydrolysis of the Panthenol leave you with the amide cutting out the need for the ammonialysis of the ester. Theres also the option of
using hydrozoic acid on the carboxylic acid to yeild the amine also IIRC.
I recall a thread here sometime back that discussed the conversion of the amide into a halogen which could be aminated in a number of fashions.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Acid or base hydrolysis of amide or esters will give the carboxylic acid. Base hydrolysis is faster.
Yikes on the hydrazoic acid idea. Azides are (IMHO) not usually worth working with due to their energetic nature. I think aminolysis is reasonable,
but unlikely to be high yielding. It is possible to do a coupling reaction using a carbodiimide (EDCI, HBTU, DCC, etc.), but you'd need to use a
moderate excess of ammonium acetate (or diethylamine or whatever) to avoid formation of dimeric esters of panthenol.
|
|
|
unome
Hazard to Others
  
Posts: 134
Registered: 17-10-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by JohnWW  | Catalytic hydrogenation or reaction with something like LiAlH4 would reduce both -OHs to Hs; while dehydration would in both cases form alkene double
bonds, offering no differentiation. But oxidation with (di)chromate or permanganate, under certain conditions, would oxidize the primary alcohol -OH
to a carboxylic acid and the secondary alcohol to a ketone, which differentiation should provide some sort of means of subsequently reducing the
ketone; although there is possibly some danger that the primary amine group could also be oxidized.
The carboxylic acid group could be esterified with glacial acetic acid plus a small amount of H2SO4, or with acetic anhydride, to reduce its
reactivity. The problem would then become one of how to reduce the ketone group to Hs while leaving the ester untouched. However, esters also undergo
catalytic reductive hydrogenation and with LiAlH4, as do ketones, so some other more selective method would have to be found. |
Hydrogenation "might" remove the secondary hydroxyl, it ain't gonna scratch the primary alcohol. I'm seriously considering looking into ways to
selectively oxidize that secondary alcohol to a ketone, then remove that with Zn/HCl.
Sedit - all the references I can find on the subject, and there are lots, all say that the product of hydrolysis is the B-alanol - which says the acid
salt would be the other product.
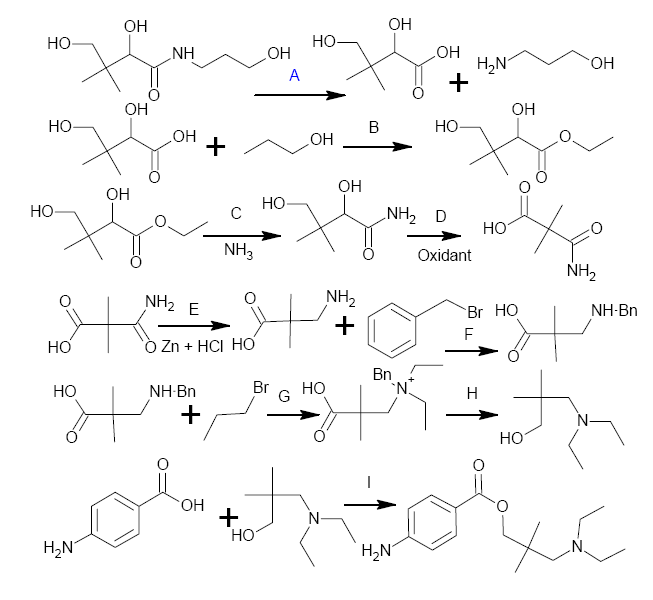
In terms of doing this with the proper equipment, anyone with access to hydrides could do this a whole lot easier
(a) hydrolysis of the amide
(b) form the ester
(c) Form the amide via the Ester
(d) oxidize both OH's to the ketone and the carboxylic acid respectively
(e) modified Clemmensen Reduction of the ketone
(f) Hoffman degradation of the amide to the amine
(g) Benzylation of the amine
(h) Ethylation of the amine to the N,N-diethylamine
(i) Reduce the terminal, primary carboxylic acid to the alcohol + debenzylation
(j) Form the ester with p-aminobenzoic acid.
Whole lot of steps, requiring lots of reagents, but starting from either panthenol or pantothenic acid (aka Vitamin B5).
Some of these steps can be skipped, if and only if, we can work out a way to avoid having to oxidize that primary alcohol in order to remove the
secondary one.
[Edited on 1-2-2010 by unome]
Someone will probably notice - I actually left the Hoffman degradation step out - instead of doing it I've changed the amide to the amine prior to the
Clemmensen Reduction, which would be daft. I'll change the pic later. Still, the point is, we need a shorter route to this.
[Edited on 1-2-2010 by unome]
|
|
|
Bolt
Hazard to Others
  
Posts: 188
Registered: 26-3-2007
Member Is Offline
Mood: No Mood
|
|
Aren't you missing a carbon after step D?
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
I see a much quicker way to this: dimethylate diethyl malonate, monosaponification followed by amidation with diethylamine (forming the acyl chloride
or using DCC), reduction with LiAlH4, esterification with p-aminobenzoic acid. Pretty staright forward.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
unome
Hazard to Others
  
Posts: 134
Registered: 17-10-2009
Member Is Offline
Mood: No Mood
|
|
Yeah, quicker by far - not so easy to source
Seriously though, I was looking through some references on hypochlorite oxidation of alcohols, secondary alcohols are oxidized selectively with
hypochlorite/GAA in the presence of primary alcohols...* Seems nice, easy, relatively simple - modified Clemmensen (I don't even want to go near ANY
FUCKING MORE Hg - I actually used to play a game with it (when I was much younger & even dumber - imagine that ), I found some old tube-lights with a small ball of it in the bottom (obviously Hg
vapor Neon type lights) and was breaking them in the air and playing with the shit ), I found some old tube-lights with a small ball of it in the bottom (obviously Hg
vapor Neon type lights) and was breaking them in the air and playing with the shit )
from that form the amide, hypochlorite again to the amine... Fuck all in terms of chemicals, all OTC, cheap and easy to source. )
from that form the amide, hypochlorite again to the amine... Fuck all in terms of chemicals, all OTC, cheap and easy to source.
The next step, forming the ethylation of the amine, that will be trickier...
* Sooner or later someone will try forming the amide from the diolacid and then try hypochlorite on that - it would probably give a flask full of
crud, might just give the imine, or even the aminoketone, thus saving some fucking around, how do you think the tertiary amine would handle Zn/HCl?
|
|
|