| Pages:
1
..
31
32
33
34
35
..
48 |
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Hi Swede - your discussion with DeNora is interesting, I wasn't aware there was any difference between bipolar and normal MMO mesh or why there would
need to be. If I recall, there was a bit of a discussion of bipolar electrodes a while back, in one of the threads.
It is my understanding that a bipolar electrode has one side an anode (facing a cathode) and electrons pass through and the other side operates as a
cathode (facing an anode). The advantage (especially to industry) is the saving on connections, contact resistance and the higher voltage (lower
current) operation (thinner cables). I guess this is an advantage to amateurs as well!
Many (more modern?) chlorinator cells are operated in a switching mode (I don't know what the timing is (seconds, minutes, days or weeks) to avoid
build up - but I wouldn't have thought it would be referred to as AC.
I have come across several 5 and 7 plate normal monopolar electrode assemblies that are operated in this switching mode. They have, of course, all MMO
electrode construction. Non-switching (polarised) chlorinators usually have just plain Ti mesh cathodes, although I did find one marked with a + and -
that was all MMO. There is a lot of variety out there!
The interesting feature of the assembly pictured above is that it is both switching and contains bipolar electrodes. I guess that once you get up to a
large number of electrodes (10) it makes sense to make some bipolar and run the cell at a higher voltage. The assembly is running in a 3 x 3 cell
series-parallel arrangement rather than 9 cells in parallel, thus requiring 3 x voltage and 1/3 current. There would be a lot of tricky spot welding
to put that above assembly together as a fully monopolar set-up!
I can't understand why a bipolar MMO electrode would need to be a different construction to a normal electrode. Possibly coated on only one side only,
but that would be more trouble than it was worth, and you could only run the cell in a non-switching mode. There would always be the possibility of
some dork assembling it back to front 
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
Deary me, I think I need to brush up on some series/parallel stuff!!!!!!!!!! (Year one, Chapter one, Page one).
US Pat. 6391176 is about a pool Chlorinator. The cycle times are shown in the picture of some text below and are of the order of 10's of seconds.
Going to another subject, his link
http://www.corrosion-doctors.org/CP/non-consum.htm
states that Pt Anodes do not like AC ripple. I guess people need to know that if using crude DC (transformer and rectifier with small or no capacitor)
on their precious Pt Anode.
Dann2

|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Hi Xenoid - I may be using the term bipolar incorrectly, and definitely using the term AC incorrectly. My limited understanding of the advanced pool
chlorinators is that on a periodic schedule, the polarity is swapped for a short time, which is supposed to clean the anode of deposits. But still,
the vast majority of its function is DC.
Denora offered what they called "biploar mesh" and it was indeed more expensive than DC-only mesh material. Either the oxides are different, the
substrate prep different, or both.
My own actual pool chlorinator appears to be interleaved solid sheets of MMO and Ti. The spacing is very tight, about 2mm. You might fit a knife
blade between them, but nothing larger.
It has been chlorinating my pool for several years now and shows no signs of wear or tear. I clean it annually in a 1/2 dilution of conc. HCl to
strip what appear to be calcium or carbonate deposits... otherwise, no maintenance required.
Best guess is that bipolar or DC-only MMO would work for chlorates so long as it is primarily Ruthenium.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Some experiments with a drip (gravity fed) HCl system...
We have discussed this in some detail in the past. I previously used a peristaltic pump with a good timer to deliver the HCl based upon an electrical
current rule of thumb. The problem was the coarseness of the delivery, and the fact that the tubes from the pump to the cell often filled with gas
between HCl deliveries, making precision difficult.
I decided to attempt a drip system, and the initial results show great promise. A drip system consists of three important components:
1) An electrically-activated solenoid valve
2) A metering or flow valve
3) A decent timer capable of good precision
Obviously too, you need a vat of HCl positioned above the system.
All of the components must be impervious to HCl, either concentrated (near 32%) or dilute. I picked up a surplus 12VDC solenoid valve that has 100%
PTFE wetted surfaces. This one was from Cole Palmer, but others would do fine. Next, an Aalborg VT-PTFE needle valve was obtained; again, all PTFE where it counts. I turned up an adapter from Teflon to connect the two, although PVDF
hose barbs would do fine as well.

The vat of "HCl" (water for now) was positioned 1/2 meter above the assembly.

Applying 12V to the solenoid triggered it to the ON state, and the water began to flow through even the narrow tubing (1/8" or 3mm ID) rapidly.
Working the excellent Aalborg needle valve allowed a very precise control, down to 1 drop every 10 seconds or so:

The only thing that is going to alter the flow of this unit is head pressure. As the HCl vat depletes, the flow rate will drop off. But
experimentation with positioning of the vat showed that the effect was very minimal. If the level of the HCl drops 10 cm, but the overall height of
the vat is at 200 cm, the head pressure will vary at worst 5%, and in reality, since the flow rate is so slow, the actual delivery variation is even
less.
The Aalborg needle valve produced a control so fine that possibly the timer itself could be skipped, and the HCl delivered on a continuous basis...
but I still prefer the concept of timed delivery.
Overall, though, gravity feed eliminates a lot of the expense and hassle of HCl delivery. One important aspect is that it must be engineered well;
otherwise, the possibility exists of a leak draining the entire vat and causing a mess or hazard, but we are only dealing with a couple of PSI or
maybe 150 millibar, depending upon the height of the vat.
The most expensive component was the Aalborg needle valve. I bought mine new for $45 US, because I could not find one used on eBay. The solenoid was
maybe $10 NIB on eBay.
A less fancy rig could be made up with cheaper components, and still get excellent results. How about a $2 pinch clamp on the tubing, setting the
system up for a continuous IV drip?
Gravity-fed HCl is the way to go! 
Cheers, & Happy new year!
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
Nice tidy job Swede.
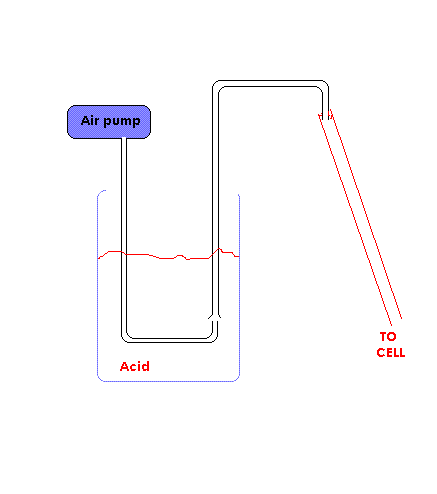
Another pump that will do this job and ideal for the cheap skate is a pump made from some tubing and an aquarium air pump. It would have to be on a
timer of course.
A diagram of the pump is shown in picture.
The pump is totally unsuitable (BTW) for pumping fluids with dissolved salts as crystals are inclined to form just above where the air enters the
'upwards' pipe. I think this type of pump is only good for pumping fluids with no dissolved solids.
Another alternative might be a simple solenoid pinching a pipe to turn acid on/off. The pipe would need to be silicon tubing so that it would be nice
and soft for pinching.
Regarding the HCl vat. If you place only enough HCl into it to do (say) one or two days, as opposed to a gallon! then if a fault developes and acid is
added to cell in one go, only one or two days worth can enter cell.
On another note.
I made some Sodium Nitrite (from Sodium Nitrate + Lead metal) to try out my Aquarium Nitrite testing kit (same as Swedes). The testing fluid is
green/blue and the test card is as pictured above a few posts.
When far too much Nitrite (way out of kit range) is added to the solution to be tested the test solution, (when dropped into) the solution to be
tested, turns dark red or purple before it reaches the bottom the test tube. When you shake the test tube the contents are purple(ish). When you wait
five minutes as stated in the instructions to let the colour develop the colour changes to a blue colour as if the PPM of the Nitrite is very very low
(zero PPM). This can be confusing. When concentrations of Nitrite are within the normal range for the kit then it works OK.
I have only tried the Nitrite kit on water + Sodium Nitrite (low ppm) + Sodum Nitrate (high concentration) + tiny amount (tint of blue) of Copper
Nitrate. The Nitrates do not interfer with the test. Will have to try it on Lead Nitrate + Copper Nitrate solution (plating solution). The blue of
the Copper Nitrate may not help as it may be difficult to see the proper colour thouht we will be diluting 1:32 (see below).
A patent states that 0.1% Nitrite reduces plating efficiency to 30% so (wild ass guess) we should try to keep below 0.01% Nitrites (100 ppm, or 100mg
per Litre). To bring this into the range of the aquarium Nitrite testing kit (5mg per litre or lower) would require the plating solution sample to be
diluted at approx. 1:32 (with water) to bring the 100ppm down to 3 ppm for the kit to test.
Dann2
[file]9391[/file]
|
|
|
Bikemaster
Hazard to Others
  
Posts: 120
Registered: 8-10-2008
Member Is Offline
Mood: No Mood
|
|
Nice set-up Swede!
I am pready sure that your gravity systheme can be easily put on a cell an it will hold the ph as steady as your dosing pump for a fraction of the
price. The only add that you should put on your systheme is a small lenght of tubing, let say 15 cm, from the end of the droping tip to the cell.
Because if you put the tip directly in the cell, salt will stick to the tip and will change the flow. I know that in your big cubic cell you have a
pipe that going in the solution, i am pready sure that this thing to can prevent salt on the tip. I think you were using it to disperce the acid, but
it will also prevent the hydrogen and chloride bubble to throw salt on the tip.
The only think i am not really sure, is that you need to put your acid tank very high in the air... I don't really feel safe with a tank on HCl over
my head that is NOT totaly close... For my systheme I think i will use the steady liquid leveler to let me put the tank less high.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Thanks Bikemaster. The plan for this thing uses an injector fitting with a length of PTFE tube (maybe 10 cm) that delivers the HCl to the interior of
a PVC diffusion pipe. The diffusion pipe is drilled with a number of very fine holes. As the acid enters, it mixes with the interior of the
diffusion pipe and is slowly leached into the cell, so there is (hopefully) no "spiking" of the cell with an acid addition.
The pipe can be seen here:

This should keep any gases or pressure inside the system from climbing up the acid tube, and the orifice is below the electrolyte level. As for vat
height, I don't think it'll take more than a meter, probably 1/2 meter would work. The only true requirement is to calibrate the system at a given
height, and determine volume of acid per minute at a given valve setting. The needle valve is so fine that one full turn of the valve barely
increases the flow, so to set up differing flows is going to require multiple turns of the valve knob.
@Dann2: Thanks for the description of the nitrite test. The little I've done with it seemed to indicate that the coloration of the copper (and/or
nickel) nitrate shouldn't interfere too much with the interpretation of the results. Perhaps the sample can be "cleansed" of Cu/Ni nitrates via some
sort of reagent that will ppt out the metals into an insoluble salt that will leave behind only a clear solution of aqueous nitrites plus perhaps
sulfates, chlorides, or whatever reagent is used to pull the colored metals from the test sample.
The other question is what level of nitrites is considered acceptable? And how do we remove them? Peroxide?
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Won't that diffusion tube tend to hold a higher concentration of HCl, resulting in Cl2 bubbling up the line?
I'd rather, say, run a tube to the bottom, then hook the end of it, so bubbles (Cl2 or H2) rise away from it.
Tim
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
My (very nice  ) diagram of a pump based on an air aquarium pump did not appear
above so it is attached. ) diagram of a pump based on an air aquarium pump did not appear
above so it is attached.
@Swede. US Pat. 2994649 gives some info. on Nitrites. They use H202 to Oxidize the Nitrites back to Nitrates. When Nitrites get to 0.1%, CE drops to
30% (Lead Dioxide plating).
I did some more testing with the kit. Added 0.012 grams Nitrite to 100ml water (approx. 120 ppm Nitrite). The amount was out of range for the kit and
when you add a few drops they sink to the bottom of the test tube and turn a purple colour in a few seconds. I do not shake the tube. The drops are
inclined to sink to the bottom (like little Lead balloons as it were).
I started to addd 35% H2O2 in 0.25 cc increments and kept testing for the Nitrites using the kit. I heated the solution and water perhaps 5 minutes
each time. I had to add 1.25cc of the H2O2 to stop getting a positive test for Nitrites. Seems a lot. The last test I done, (zero Nitrites as they
were all now destroyed by H2O2) I shook the test tube and waited for colour to develop but I did not get a blue colour as I should have but rather a
green colour. I don't really know what this means. There is no green colour on test card.
I guess we will have to wait for when an LD plating session begins to really see if kit will work in an LD plating solution.
I had thought/hoped that Methylene blue might detect Nitrites in water. Excess of Hydrogen Peroxide giving a blue colour and excess of Nitrite
(reducing conditions?) would give a clear colour but this does not work at all. I used an acidic (some Nitric acid) solution to try it out. Mehylene
blue is a REDOX indicator BTW.
I cranked up a Perchlorate cell approx. 3 weeks ago using (whats left of it) the Ti substrate LD Anode. It has been sitting around for approx. six
months. The Anode works OK, no passivation between the LD and the Ti. Will let it go for a month and pull the plug. I will be doing no testing etc, I
am just seeing will the Anode continue to work.
I am getting around to setting up a Chlorate cell with a large and a little Cathode. Will use pH controll. When cell has stabalized (constant amount
of acid addition to keep pH at around 6.7) I will swap in and out the bigger/smaller Cathodes each 24 hours and see how much the CE changes by doing
some titrations.
Dann2
[Edited on 12-1-2010 by dann2]
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by 12AX7  | Won't that diffusion tube tend to hold a higher concentration of HCl, resulting in Cl2 bubbling up the line?
I'd rather, say, run a tube to the bottom, then hook the end of it, so bubbles (Cl2 or H2) rise away from it.
Tim |
That's a good point Tim, and I hadn't really thought about it. I was hoping that evolved chlorine would dissolve and become aqueous, but it is worthy
of consideration.
Wherever the conc. HCl contacts the liquor, you are going to have evolved chlorine. If using timed doses, it'd be nice to either very slowly add the
dose, or add it in such a manner that it gets diluted very rapidly.
My new cell has mechanical stirring via a motor and a Ti paddle. Perhaps the best place for acid addition would be right next to the rotating paddle
for instant dilution.
|
|
|
Leander
Harmless

Posts: 28
Registered: 23-2-2008
Member Is Offline
Mood: No Mood
|
|
I was planning to make Ba(ClO3)2 by electrolysis of BaCl2 solution. I have some sources indicating that barium salts (along with cadmium) shouldn't be
used with MMO anodes. Can anyone confirm this, and is there any logical explanation?
|
|
|
Contrabasso
Hazard to Others
  
Posts: 277
Registered: 2-4-2008
Member Is Offline
Mood: No Mood
|
|
A research document I have suggests that Barium has adverse cathode zone reactions and needs careful treatment.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
Would it be possible to give us links to the sources that say Cd and Ba are harmful to MMO and by all means upload the research document on Barium
Chloride.
I have also read that Ba is harmful to MMO. How harmful I don't know. It's on my page.
TIA,
Dann2
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
I bubbled air through a solution (0.35 grams in 640ml water) of Sodium Nitrite for 24 hours using a plastic 'air stone' and a tall vase. The solution
still gives a similar positive indication of Nitrites (using the Aquarium Nitrite detection kit) to when bubbling was started. Bubbling air through a
plating solution to get rid of Nitrites in a non starter IMO.
There is some info. on Nitrites at this link
http://library.sciencemadness.org/library/books/Mellor_ACTIT...
on page 459, if anyone is interested.
Also see this link:
http://www.nature.com/nature/journal/v133/n3354/abs/133213b0...
I think it may be possible to lower the Nitrite concentration of fairly concentrated solutions of Nitrite by bubbling air but the very dilute
solutions (of Nitrite) we will be dealing with are not going to be lower in concentration by air bubbling. See Mellor.
Dann2

|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Maybe we can use some nitrobacter to oxidize it for us?
Or maybe not... 
Tim
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Have you tried peroxide yet? Or how about bubbling ozonated air? There are a number of fairly inexpensive low-dose ozonators available (yet again)
for the aquarium trade.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
I have tried H Peroxide in my post of 11 Jan. It seemed to take alot of Peroxide to destroy the amount of Nitrite present.
Just wondering, when LD plating is taking place and their are Nitrites present (what we want converted back to Nitrates) does the H Peroxide we add
act directly on the Nitrites or does it perhaps involve some reaction at the Anode involving the Nitrites + the H Peroxide???????
Wild assed thought, my chem's not red hot............
I will check out the Ozonators. Sound like something that could work. I do not love the idea of bubbling air/gas into an LD plating set up because of
the extra splashing/mist it may create but I guess a good cover on the tank would deal with that end of things.
On a different note, just wondering if something like cabosil, or powdered aeroboard or floating beads were placed into a Chlorate or Perchlorate
cell, would it stop the terrible mist (Satans breath, that rusts all in sight)?
I have attached a good read on making K Chlorate from K Chloride using Lead Dioxide Anode. Thanks to Solo over in references. I have never seen the
article before.
They state that coating Graphite Anodes with Lead Dioxide makes the Graphite Anodes last at least three to four times longer!!! Marvelous!!
They used 2N HCl (7.3%) for pH controll.
Dann2
Attachment: Lead_dioxide_anodes_in_the_large_scale_production_of_potassium_chlorate_from_potassium_chloride.pdf (234kB)
This file has been downloaded 1179 times
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Until I get my LD plating rig set back up, I can't help with the nitrite issue. The used liquor from the last run probably will be altered from what
it was... it's been almost a year.
I agree with the thought that any bubbling in the bath is a really bad idea. There's enough gassing from the electrodes. We don't need to add to it
with hard, rolling bubbles from an aquarium pump.
I am excited to give my mechanical stirring rig a shot on my next run. I have extensively modified my "T-Cell" using what I've learned, and the next
major run will consist of mechanical stirring, no lousy bubbler to jam, and a gravity-fed HCl system. Since the cell is opaque, I set up a "dip
stick" method to check the liquor level. I trimmed a glass pipette, added a PTFE collar, and to check the level, all I have to do is close off the
end of the pipette and remove it from it's fitting. The liquid will be trapped, and I can compare it to a pre-made scale.
I have about 300 cm^2 of Pt mesh coming. I haven't given up on LD, but I do want to work with Pt as well. A couple of miniature test runs with a
small Pt anode produced beautiful results. I need to scale it up a bit.
Nice paper, dann2, it's got some good info on it. Thanks. Right into my electrochemical PDF collection it goes.
[Edited on 15-1-2010 by Swede]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
Pt is the King of electrodes. The poor mans Pt takes some time, effort and less cash............ I think.
Attached is a paper on a pilot scale plant for the production of Ammonium Perchlorate in Europe.
Have not seen this myself before.
It came from this link:
http://www.nt.ntnu.no/users/skoge/prost/proceedings/ecce6_se...
Have a good read.
Dann2
Attachment: Ammonium Perchlorate pilot plant_2007.pdf (520kB)
This file has been downloaded 1137 times
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
Keep reading.
Attached is a must read on Potassium Perchlorate (and Sodium Chlorate and Perchlorate) manufacture.
I have not come accross this paper before.
Thanks to Solo.
Some salient points (IMO, everyone will have their own):
A good laugh at the top of page 64.
They check pH and add acid to the Chlorate cell every 24 hours only  for to
contoll pH. for to
contoll pH.
The Chloride content is reduced to 84g per litre with Graphite Anodes.
The Chlorate content is reduced to 20g per litre with Pt.
pH not controlled in Perchlorate cell stage and goes to 10.5.
What is 1:1 and 1:2 HCl acid? Is it 36%HCl diluted with one parts water and two parts water?
Cheers,
Dann2
Attachment: Production_of_Potassium_Perchlorate.pdf (673kB)
This file has been downloaded 886 times
[Edited on 16-1-2010 by dann2]
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Another interesting read. They have the batch process down pretty nicely, considering it was 1942.
| Quote: |
What is 1:1 and 1:2 HCl acid? Is it 36%HCl diluted with one parts water and two parts water?
|
I think we can be confident that that is exactly what it is, 32 to 36% commercial HCl diluted 1:1 or 1:2.
They use tubular cathodes and axial Pt rod anodes for the perchlorate cell. I found this to be interesting:
| Quote: | | Operation of the cell is greatly affected by the chloride content of the cell liquor. At concentrations of more than 2% (dry basis) no perchlorate is
formed. The effect is abrupt, and it appears that a critical chloride concentration is involved. No explanation of this effect can be offered.
|
In other words, Pt does not, and never did, like chloride. I know Pt is supposed to work all the way from chloride to perchlorate, but something
doesn't ring true, and this document supports the notion: "Use LD/Pt for perchlorate. Use MMO or graphite for chlorate." A one pot process from
chloride to perchlorate is going to be very difficult, materials-wise. Perhaps LD can do it, but given the availability and low cost of both graphite
and MMO, it makes little sense to risk the precious perchlorate anodes on chlorate.
Getting a harvest of chlorate that is well below 2% chloride is not difficult at all, and that is your feedstock for the perchlorate cell.
Additives: They use dichromate in the chlorate cell to prevent cathodic reduction. Is there an additive recognized to be helpful in a Pt-based
perchlorate cell to do the same thing? We don't want our oxidized products reduced to chloride, which will increase anode erosion and slow or inhibit
the process.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Swede,
They actually use Dichromate at (approx.) 1 gram per litre in the Perchlorate cell. See page 57.
With LD you cannot use Dichromate so Flouride seems to do a similar job. Persulphate as also been used in LD cells.
One problem I have with coaxial arragements (pipe and rod) it that you are forced to have a large Cathode area in relation to Anode area. This may
have serious implications if running cells with no additive (green cells) to stop Cathodic reduction reactions. Large Cathode area ==> large amount
of reduction AFAIK and no additive to stop it. Four or five wires in parallel with the rod would be good if using a green cell. Less area and still a
good current distribution on Anode.
The cells are actually not bipolar even though they look like they are since a 'comb' arrangement is used. All Anodes are actually physically
connected to the + of supply with 3.1-3.5V accross cell.
I think the 'Holy Grail' method of making Perchlorate should perhaps be renamed the 'Unholy' method. Far too much time and wear on the precious Anode
(Pt or LD) during the Chlorate stage and if you are not using pH controll during the Chloate stage then it's simply crazy.
As stated by yourself and others, seperate the two processes.
Bit of a paper splurge (with more to come). This one discusses some history. Some interesting 'garage like' pictures of early Perk making stuff.
Dann2
Attachment: History of establishing K and Amm Perk sources.pdf (916kB)
This file has been downloaded 935 times
[Edited on 17-1-2010 by dann2]
|
|
|
Lambda
National Hazard
   
Posts: 566
Registered: 15-4-2005
Location: Netherlands
Member Is Offline
Mood: Euforic Online
|
|
Reading Material
Chlorates and Perchlorates.rar (15.5 MB) contains the following Files:
1. - Lead Dioxide Anodes - HTML
2. - US Patent 5.104.499 - Electrolytic Production of Alkali Metal Chlorates and Perchlorates.pdf
3. - Chlorates and Perchlorates.pdf
4. - An Idiots Guide to Making Chlorate's and Perchlorate's - By WarezWally.pdf
5. - A Study of the Production of Sodium Perchlorate Electrolytically - By Frederic A. Maurer (Thesis - California Institute of Technology - 1923)
33s.pdf

6. - Materials Handbook - A Concise Desktop Reference - 2nd Edition - By François Cardarelli (Springer-Verlag - 2008) 1365s - Including Bookmarks
* Lead Dioxide (PbO2) Electrodes; Starting at Scan Page 606 with Preparation and References on Scan Page 607
iFile.it Download Link:
http://ifile.it/35xrnhz
No Password Required !
Enjoy !
Lambda
|
|
|
Contrabasso
Hazard to Others
  
Posts: 277
Registered: 2-4-2008
Member Is Offline
Mood: No Mood
|
|
While Pt seems not to like going through from chloride to perchlorate in one step, there was a cell in the UK running a lead dioxide anode from
saturated chloride to perc. The guy did loads of write ups on his own www with all the testing and the first run. IIRC he ran it at 80a continuously!
Then a UK supplier offered him a drum of perc so he stopped playing. He's been very ill since and not maintained the www sadly.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Pt Porn
My long-delayed raw 3 micron Pt over Ti mesh finally arrived. I absolutely have not given up on LD and other anode attempts, but I did want some Pt
mesh to experiment with.


Pt is expensive - in any form. A reagent-grade salt to pure Pt metal; it doesn't matter. I think in the end, it would cost as much to plate an anode
with an equivalent Pt coating beginning with a Pt salt, as simply buying this material from the start. The Pt load of this mesh is 50 grams per
square meter.
I am probably going to craft tubular Ti shanks for these, filled with Pb, to fit a 1/2" PVDF compression fitting.
|
|
|
| Pages:
1
..
31
32
33
34
35
..
48 |