12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Smelly on a whole new level?
Alright, amines, thiols and especially isocyanides, are renouned for their pungent odors. A few others as well, skatole for instance. But has anyone
combined them on a single substrate to make some superstinker?
Are these groups compatible, would the molecule rearrange? (Who wants to find out?!  ) )
Tim
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Beware that if you get the molecular weight high enough, it won't smell like much anything, as it won't volatilize enough. For this tidbit and much
more on the subject, see Luca Turin's book The Secret of Scent. There's a lot there to inform what might happen when you combine smelly
moieties, in particular his hints on how changing electron density on a bond can change perceived smell.
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
I think that from that thread on thioacetone, I'd give it my vote for unholy king of reek without needing to combine groups. It's mind-blowingly
potent and has a low enough molecular weight to be nice and volatile.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
HydroCarbon
Hazard to Self
 
Posts: 77
Registered: 7-7-2008
Location: Anytown, USA
Member Is Offline
Mood: No Mood
|
|
Good or bad, I have to say that smells are one of my favorite parts of organic chemistry. It really is amazing how many different scents you can
encounter, and how some can be AMAZINGLY potent. Off topic, but in my rather limited experience with organic I have to say cyclohexene was one of the
most interesting scents I've encountered; an almost metallic sharp scent if I recall correctly.
I also recall when a classmate opened a bottle of some organic reagent, I don't remember what it was, he was trying to synthesize a cockroach
pheromone; but just opening the bottle caused the whole lab and the hallway outside to stink like dirty socks.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
One ponderance: why does thioacetone smell so badly, while thiourea doesn't smell at all? (Well, I don't have a pure sample, but a mild solution
certainly doesn't smell odd.)
I suppose, to answer that, you first have to look at ordinary urea, which is solid and more-or-less odorless. And fundamentally, urea is solid
because of hydrogen bonding on the amines and the polarity of the amines and carbonyl. Is it generally true that hydrogen-bondable chemicals tend not
to be stinky?
Acetone is as good a substrate as any. Ordinary acetone is volatile with a mild ether odor. Thioacetone is satanic. Chloracetone is terribly
irritating (although not necessarily odorous, but that's probably due to burning away the smell receptors..). What about acetone isocyanide? Would
anyone dare risk thioacetone isocyanide?
Tim
|
|
|
Pyrovus
Hazard to Others
  
Posts: 241
Registered: 13-10-2003
Location: Australia, now with 25% faster carrier pigeons
Member Is Offline
Mood: heretical
|
|
Combining amines with thiols would likely be counterproductive, given the acidic nature of the S-H group and the basic nature of the amino group,
there would be a significant tendency towards proton transfer. As such, there would be an equilibrium with some quantity of zwitterion present,
consequently reducing the overall volatility of the substance.
Never accept that which can be changed.
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
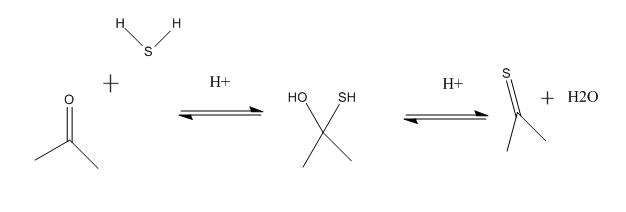
The thioacetone topic piqued my curiocity, so I tried to make some ... in an area remote from where I live ... by acidifying acetone and adding a lump
of ferrous sulphide.
Hydrogen sulphide was liberated, but unfortunately, or maybe fortunately, the reaction that I expected did not happen. Nor did pyrolising the mixture
by painting it onto a piece of hot tin.
|
|
|
Ramiel
Vicious like a ferret
  
Posts: 484
Registered: 19-8-2002
Location: Room at the Back, Australia
Member Is Offline
Mood: Semi-demented
|
|
I'm not sure H2S is such a good nucleophile so as to induce an attack by the proposed SN type mechanism, is this right?
Caveat Orator
|
|
|
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Such reactions do occur at a useful rate but it varies of course based on what ketone you are using. Sulfur is almost always a good nucleophile after
all unless the electrophile is fairly hindered. I don't have the papers handy but I was looking at this reaction and others not too long ago (maybe a
year) regarding the manufacture of thiuram monosulfides. My goal was to push it further and make geminal thiols in the same way that one can make
aminals from secondary amines and a ketone.
Regarding the rest of the thread. It is interesting to think of the way these smells would meld if it were not for the chemical reactions that might
occur between the functionals during the mixing process that could ultimately diminish the potency. Isocyanides and amines are often mixed, the
preparation by dehydration of a formamide using phosgene has plenty of TEA in there as an acid scavenger. Given TEA isn't the worst of smells but it
doesn't seem to add much the bouquet of the crude material.
Some chemicals have deceptively smelly names. I worked with cadaverine before and it just had that amine smell. It wasn't filed in my mental rolodex
that 1,5-pentanediamine was the same chemical. I've also had the pleasure (as I've related elsewhere on this forum) to behold the series of thiols
from ethanethiol all the way up to dodecylthiol. I was doing a lab pack project at a university and the leaving chemist had done research on the
bunch. It wasn't horrid but then again I didn't open any of the bottles. It was interesting the levels of stink of each individual unit and then the
odor when they were combined.
Although it was a mixture of odors, the worst smell I've ever beheld was skunk. Specifically a skunk that had died at the bottom of a crock on a
trailer. In this instance, a crock on a trailer for those of you uninitiated is a plastic tub approx. 3 feet in diamter sunk probably 8-10 feet into
the ground. From this tube the water for the trailer comes up out of the ground to meet with the trailer as a 2 inch tube. The purpose of the crock
as I've been told is just to connect to the water and make sure things don't freeze.
To get to the juicy details sometimes these things fill with water. In this case there was a foot or maybe a foot and a half of water in the crock
and a skunk had managed to get in there and die. Dead skunk is one thing but give it a few weeks in the summer to fester first. I was on the job
when the owner of the trailer called and complained about the smell. My boss at the time took his crew over there and deduced that something had died
in the crock so the plan was to pump it out. We dropped the feed for the pump into the crock and turned it on. At first horrible smelling water came
out, then chunks of the skunk. The smell was so overpowering that I immediately threw up. Then everone else threw up. We tried to get up wind but
it wasn't that windy. Tried to spray it down with water. All in all I threw up twice. My second time is when we pulled the pump out when it was
plugged with bone and skin and fur. Now that was a stench.
[Edited on 12/12/2009 by BromicAcid]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Paddywacker: Thats very brave of you... I would suggest using P2S5 as the sulfurating reagent... I'm almost certain (99.5%) that it would work. Whilst
thiols (of which H2S can be considered a member: H-SH) can be reacted with carbonyl groups, the product is the dithioacetal (I guess H2S isn't quite
as analogous as I'd like here...); in most instances I have seen, BF3.Et2O is used as the catalyst, instead of a protic acid.
|
|
|