libavius
Harmless

Posts: 9
Registered: 3-5-2009
Member Is Offline
Mood: No Mood
|
|
Mellitic acid
Anyone knows the method to produce (oxidize) the carbon to Mellitic acid?
I had read it somewhere but now I can not find the procedure.
Thanks!
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
I have read somewhere of the stuff, formula C6(COOH)6, being made by oxidizing graphite or hexamethylbenzene with hot concentrated HNO3 or alkaline
KMnO4. This has been in a thread here somewhere before. It was first discovered in 1799 in the mineral mellite (honeystone), which is the aluminium
salt of the acid, and which is produced when strongly acid waters caused by oxidation of sulfide minerals buried in clays, thereby containing Al in
solution, come into contact with coal.
The free acid was first prepared by warming mellite with ammonium carbonate, boiling off the excess of the ammonium salt and adding ammonia to the
solution. The precipitated alumina is filtered off, the filtrate evaporated and the ammonium salt of the acid purified by recrystallization. The
ammonium salt is then converted into the lead salt by precipitation with lead acetate and the lead salt decomposed by hydrogen sulfide.
Its anhydride, C12O9, obtained by heating the acid, contains no H and is so an oxide of carbon.
For further info about its properties, see http://en.wikipedia.org/wiki/Mellitic_acid and http://en.wikipedia.org/wiki/Mellitic_acid
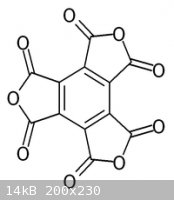
[Edited on 22-11-09 by JohnWW]
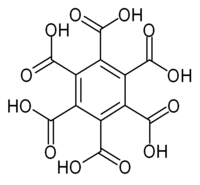
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Electrochemical oxidation of graphite anode in deionized water at galvanostatic mode is investigated. It is found that mellitic acid and graphite
oxide colloid (>20 nm) are coexisted as main products, as identified by XRD and TEM, FTIR and XPS. They can be separated successfully through
several procedures. A few amounts of graphene and graphene rolls are also appeared as co-products. |
http://dx.doi.org/10.1016/j.elecom.2008.12.003
http://www.osti.gov/energycitations/product.biblio.jsp?osti_...
Mellitic Acid from the Oxidation of Graphite with 90% Nitric Acid.
http://pubs.acs.org/doi/abs/10.1021/je60022a027
Mellitic Acid from Coals, Cokes and Graphites
http://pubs.acs.org/doi/abs/10.1021/ja01280a052
somewhat related
PYROMELLITIC ACID
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv2...
also use this site's search function, I believe that the topic has been discussed before.
|
|
|
libavius
Harmless

Posts: 9
Registered: 3-5-2009
Member Is Offline
Mood: No Mood
|
|
Thanks JohnWW, thanks Not_important, you were very useful for me.
I knew that was a synthesis difficult, but I expected a better yield!
Perhaps the one only slightly executable will Pyromellitic acid.
Bye
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
And how would one best reduce this to the cyclohexane version (hexacarboxycyclohexane)?
Birch reduction - not even sure if that would work, and in any case one would get the diene version.
Looks like metal catalyst, i.e. Pd/H?
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
Using rhodium as the catalyst (or still plain Pd but with really high pressure) would definitely reduce the benzene ring to cyclohexane; as to whether
the carboxyls will survive that treatment unreduced (to the alcohol, that is) is what I ain't too sure about.
sparky (~_~)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|