galvanize
Harmless

Posts: 3
Registered: 2-5-2008
Member Is Offline
Mood: No Mood
|
|
trichloracetimidate
Hi all,
I need to synthetize methyl 2,2,2-trichloroacetimidate, I saw the synthesis from Cl3CCN, but, I only have 2,2,2-trichloroacetic acid to start the
synthesis.
Can anybody help me please?
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
Are you sure you don't mean trichloroacetamide? Trichloroacetamidate would be an anion.
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Liebigs Annalen der Chemie (1981) 70-84.
Chemische Berichte, 91 (1958) 1049-1054.
Journal of the Chemical Society [Section] C: Organic (1968) 1479-1483.
Phosphorus and Sulfur and the Related Elements, 35 (1988) 1-4.
Environmental Science and Technology, 24 (1990) 81-86.
Berichte der Deutschen Chemischen Gesellschaft [Abteilung] B: Abhandlungen, 64B (1931) 661-667.
Trichloracetamino-Methyl Ether. Berichte der Deutschen Chemischen Gesellschaft, 40 (1907) 1643-1646.
| Quote: | | I only have 2,2,2-trichloroacetic acid to start the synthesis. |
Then buy the nitrile.
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
Maybe heating with urea will do. Heating GAA with urea yields acetamide.
|
|
|
galvanize
Harmless

Posts: 3
Registered: 2-5-2008
Member Is Offline
Mood: No Mood
|
|
I mean methyl 2,2,2-trichloracetimidate
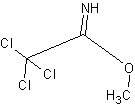
|
|
|
grind
Hazard to Others
  
Posts: 120
Registered: 13-1-2007
Member Is Offline
Mood: No Mood
|
|
I think it is necessary to prepare the amide, which is the precursor of your temporarely desired nitrile.
Maybe it´s possible to go the usual way, that means adding SOCl2 to your acid to obtain the (when I remember correctly, very volatile) acid chloride.
With the chloride at hand, it should be easy to prepare the amide.
An important fact is, you have to find conditions which avoid the decarboxylation of the trichloroacetic acid to CO2 and CHCl3.
There are a lot ways from the amide to the nitrile, literature search will help you (similar prep. of amide).
Ok, all this are of course presumptions, I didn´t do any research concerning your problem. So don´t beat me if my presumptions are wrong  ... ...
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
If only you could form imines of esters... it might be possible, but I'm not aware of it! Even then you would have problems with ester hydrolysis.
|
|
|