| Pages:
1
2 |
qton
Harmless

Posts: 13
Registered: 19-9-2009
Member Is Offline
Mood: No Mood
|
|
Recommended Microscope Stains?
I'm looking at buying some stains for microscopy (bacteria and cell). Since I'm working out of home, I'll need to stick to Relatively Safe Stains (No
kids, but the wife fears exotic poisoning ;-). Does anyone have comments on the following list? Are there any safe or relatively safe stains worth
adding to the list?
--- Relatively Safe Stains
Eosin Y, certified C.I. #45380
Methylene blue, certified C.I. #52015
Nigrosin, w/s C.I. #50420
Basic fuchsin, certified C.I. #42510
-- Safe, but probably not much use
Rose Bengal, certified C.I. #45440
--- Could get these, but look risky...
-- Gram's Stains (very useful, but hazardous)
Gentian violet C.I. # 42555 (carcinogenic)
Gram's iodine (mutinagenic)
-- Toxic (eye hazard)
Safranin O, certified C.I. #50240
-- Very Useful, but Very Toxic
Haematoxylin, certified C.I. #75290
Neutral red, certified C.I. #50040
Questions:
1. These ingredients are the powders, so I would have to make the solutions myself. Googled around and this doesn't look that hard. Would you agree
with this?
2. The MSDS make for scary reading. Assuming I take the appropriate precautions, can I handle these substances safely (ie. without getting cancer.
;-)?
* Gram's stain would be very useful, but the MSDS are scary to say the least.
* So would Haematoxylin, but again the MSDS makes for sobering reading.
* Is the risk of eye damage from Safranin O so severe I should avoid using it?
3. Been looking for a mounting medium to make permanent slides. Most popular seems to be ENTELLAN but it's a hazardous substance so expensive to mail
order (and as usual, toxic as hell). The other mounting medium cost $$$ and the minimum order is vat-size. Are there any alternatives to these? (like
dehyrating the specimen with alcohol and then just aralditing the corners of the cover slip?)
Thanks!
Useful MSDS site: http://msds.chem.ox.ac.uk/msds-searcher.html
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
The most important problem is when you open the bottles, they often release a puff of dye dust - and THAT you do not want to inhale.
Most dyes aren't healthy, even fluorescein, when consumed, is not good for you, so I'd always use caution. Best do outside or in a fumehood.
Some dyes aren't water soluble, so you need to check for that, others are only soluble as salt (i.e. fluorescein), others yet again are only soluble
in acid. Then you have all those dyes that are only solvent soluble...
For making permanent slides, you could start looking into castable polymers - these can be diluted, and a resin forms. Or fix with glutaraldehyde,
formaldehyde etc, that will make the samples already quite resistent to degradation.
Have a look at this also
http://en.wikipedia.org/wiki/Staining_%28biology%29
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
qton, I have been using all those microscopic stains for at least 45 years and have not found them to be at all hazardous when used sensibly. My main
objection to them is that weighing them out coats surfaces and clothes with the dye particles and stains them beyond repair. I have had all of them
on my hands and the only problem is removing the multi-colored spots. Wearing gloves will prevent skin contact and is recommended these days.
I never knew that Safranin was an occular toxin. I have eye problems but they are all due to aging, according to my eye doctor.
But I must admit I have never eaten these dyes or put them in my eyes. Common sense should be enough, but I second Chemoleo's advice not to breathe
the powders. You can buy them already in solution and avoid that risk.
[Edited on 20-9-2009 by entropy51]
|
|
|
crazyboy
Hazard to Others
  
Posts: 436
Registered: 31-1-2008
Member Is Offline
Mood: Marginally insane
|
|
I have found methylene blue to be the most useful stain by far.
|
|
|
qton
Harmless

Posts: 13
Registered: 19-9-2009
Member Is Offline
Mood: No Mood
|
|
Thanks very much for the warnings, chemoleo. I hadn't thought of the puff of dust. I had planned on making the solutions outdoors. Aside from goggles
and a mask, I could uncap it in a plastic bag?
Thank you entropy51. After trying many chemists and getting blank stares, I finally found a methylene blue and malachite green stain in - of all
places - the local supermarket. Fish parasite treatment. Turns out the malachite green MSDS is the scariest of the lot, but crazyboy is right: turns
out it makes a nice stain after all. :-) Thanks also for the warning about the bits of dye everywhere: I guess you can cover the measuring scales and
not make solutions wearing your Sunday best. :-)
I find the MSDS say a lot of things, and don't always make sense. Sometimes a carcinogen can have a health rating of 1, but I guess it's all relative.
They all agree Safranin O is something to squirt in your eye , but as you say use common sense (and spash goggles) and you'll be okay.
For years I've been cultivating yeast (okay... brewing beer) and using with Sodium Metabisulfate. Scary MSDS too (but it was worth it ;-)
http://www.jtbaker.com/msds/englishhtml/S4378.htm
[Edited on 2009-9-21 by qton]
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Since when is Hematoxylin dangerous? I've dyed fabric numerous times with logwood, which is the source of this dye with absolutely no ill effects.
http://www.jtbaker.com/msds/englishhtml/h0304.htm
Neither does neutral red seem to be any particular hazard. I wouldn't eat the stuff, but it seems tame otherwise.
http://www.jtbaker.com/msds/englishhtml/n2550.htm
I have @3kg of metabisulfite on hand at all times. It's mostly harmless except for the smell. Sulfur dioxide is one of my least favorite gases.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
qton
Harmless

Posts: 13
Registered: 19-9-2009
Member Is Offline
Mood: No Mood
|
|
| Quote: | [quote=163444&tid=12837&author=UnintentionalChaos]Since when is Hematoxylin dangerous? I've dyed fabric numerous times with logwood, which is
the source of this dye with absolutely no ill effects. Neither does neutral red seem to be any particular hazard. I wouldn't eat the stuff, but it
seems tame otherwise.
|
It's very hard to judge this stuff. MSDS err on the size of safety (... from law suits?) I noticed the other day that the wool wash in our laundry
says 'seek medical advice if splashed in eye' or something like that. I guess that means it's an ocular poison, but they don't even list the active
ingredient on the side let alone have an MSDS.
Occasionally seems they do discover something in common use turns out to be mutinogenic or carcinogenic. But so is peanut butter if you leave it long
enough. It would be much better if there was a single source for MSDS: they seem to be per supplier, vary wildly and don't quantify the risks so you
can't judge them.
| Quote: |
I have @3kg of metabisulfite on hand at all times. It's mostly harmless except for the smell. Sulfur dioxide is one of my least favorite gases.
|
A couple of years ago I visited a volcano crater arriving just as it belched up a big cloud of the stuff. Felt a stinging pain in my lungs, and it was
really hard to breathe. Not labored; I just couldn't breathe at all. Like my lungs just stopped working. Not recommended.
[Edited on 2009-10-11 by qton]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
| Quote: |
Turns out the malachite green MSDS is the scariest of the lot,
|
That surprises me. We made this dye in organic lab and then proceeded to make tie-dyed shirts with it. The instructor never said boo about toxicity.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Quote: Originally posted by entropy51  | qton, I have been using all those microscopic stains for at least 45 years and have not found them to be at all hazardous when used sensibly. Common
sense should be enough, but I second Chemoleo's advice not to breathe the powders. You can buy them already in solution and avoid that risk.
|
I guess you can either believe this crazy old guy or you can believe the MSDS.
Every MSDS is scary. You shouldn't completely ignore them, but you should also look at other sources of information. Talk to a
microbiologist at school or in a local medical laboratory. They use those stains all the time and can give you a realistic appraisal of the risk, if
any.
Every MSDS is scary because all chemicals have some toxic property under some circumstances. That's why you need to handle them properly, which is
most cases is just common sense in avoiding unnecessary exposure of you to them. If you buy the prepared solutions, wear gloves and don't spill them
on yourself, your exposure will be negligible. These dyes are not volatile and you will not breathe any fumes.
If you're scared of biological stains just go buy some food coloring in the grocery store. If you experiment with them you may find that they stain
bacteria or other cells. It won't be a Gram stain, by any means, but if they're safe for food use surely you can stop worrying about miniscule
hazards of real stains.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Even the MSDS for table salt is scary. You need gloves and eye protection if you need to handle this compound.
The problem with MSDS's is that they are written with the most extreme thing in mind, which COULD happen theoretically. If you are handling table salt
in tonne-quantities, then of course you need some protection against dust in your eyes and on your skin. Salt can be very irritating. This all is
written with industrial uses in mind, in bulk quantities. As entropy51 stated, you should not ignore the MSDS-sheets, but you must keep in mind that
your pattern of use and possible exposure is of a totally different order than what the authors of the MSDS-sheets have in mind.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
The most dangerous biological tissue stain for preparation of microscope slides of which I have heard is OsO4, formed by heating Os metal in air and
condensing the tetroxide. It has a penetrating odor, and is highly toxic and volatile, attacking eye and mucuous membranes. On contact with biological
tissues, it is reduced to the colloidal metal, which is very dark in color. However, I doubt that OsO4 is used much now, because of its being so
hazardous to use, and very expen$ive. The stuff is also used in organic chemistry for oxidative cleavage of C=C bonds in laboratory syntheses, in
situations where use of KMnO4 solution for the purpose would be undesirable.
Aqueous AgNO3 and Ag2SO4 could be used as a less dangerous and much less costly alternative to OsO4 for the specific microscopic staining purposes
involved, which call for tissues to be outlined or coated in a dark opaque metallic material. Ag(I) salts in solution are also reduced to the very
dark finely divided colloidal metal on contact with biological tissues.
[Edited on 12-10-09 by JohnWW]
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Quote: Originally posted by JohnWW  | | The most dangerous biological tissue stain for preparation of microscope slides of which I have heard is OsO4, formed by heating Os metal in air and
condensing the tetroxide. It has a penetrating odor, and is highly toxic and volatile, attacking eye and mucuous membranes. However, I doubt that it
is used much now, because of its being so hazardous to use. |
OsO4 is still used, but that's for
electron microscopy and that's not what qton is asking about. (My histology professor used to joke about how many times she had mouth
pipetted OsO4 as a grad student!)
|
|
|
qton
Harmless

Posts: 13
Registered: 19-9-2009
Member Is Offline
Mood: No Mood
|
|
Thanks everyone for your advice. Took me a while, but I've finally got some stains done. (I used a Methylene Blue Malachite Green stain (because
that's what the pet store sold :-). Took so long because I didn't know how to stain. Flooded to get some great cheek cell results, but flooding worked
miserably for everything else. Eventually found a copy of Prescott Microbiology and realized I needed to do this: Smear/Air Dry/Heat fix/Stain/Wait 5
minutes/Washed with Water/Air Dry.
For the staining I used precautions like latex gloves and a mask /goggles to protect against an accidental splash. Avoided skin contact and mixed
outdoors. Seemed fine. I'll mail order some of the other stains. Thanks again for your advice about handling. Without being able to watch someone
doing this in lab, it's trial and error and the scary MSDS don't exactly inspire bravado. But I get the idea now: read the MSDS just in case and take
common sense precautions.
These are my saliva and nasal mucus (Sorry!) So can anyone tell me what I'm looking at. Surprisingly few "What's that Bacteria!" web pages on the net.
Have ordered a couple of microbiology textbooks, but until then can anyone tell me what these are?

#1: Saliva 1000X. Previously looked at Saliva with 100X darkfield and that was pretty cool. You can see little dots rocketing around which I presume
are bacteria. There are longer thin lines which seem to drift. Not real sure what they are. This *isn't* the darkfield but an MB/MG stain. I presume
the little cyan (they were green - excuse the crap microphotography) dots in the center are bacteria? What's the other stuff?
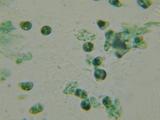
#2: Nasal Mucus 1000X. What the heck are the green spheres? If they're bacteria, they're much larger than the bacteria in #1. White blood cells maybe?
Have had a nose infection so was expecting to see bacteria, but if that's not them they're MIA.

#3: Same 100X.

#4: Same 400x. Note what I presume is a cell from my nose lining.
BTW regarding the electron microscopy LOL yeah I wish but Slashdot did report this: http://science.slashdot.org/story/09/10/11/1143247/An-Electr...
[Edited on 2009-10-18 by qton]
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Very nice! The first one - why do you think these are bacteria? It could equially be cell debris (membrane, protein, polysaccharide aggregated mucus).
Bacteria are normally very small regular blobs that float everywhere, of equal sizes.
The remainder - very nice sample of epithelial cells - from your nostril.
WHat cameraµscope did you use? Can't be so cheap with the camera attached?
The cell treatment of yours - you change of course the properties (look, size, aggregation behavior etc) of the cells by heating and drying them.
Best is if you can make the cells attach themselves to a hydrophobic surface (plastic instead of glass slides) so that they stick - then you can
incubate them with whatever you want, and wash it off after. I.e. take up your mucosal scrapings in PBS buffer with a bit of glucose, let them sit on
a plastic slide for a while (6 hours, overnight), wash off everything then and see if something stuck to the slide. If so, carry on with the staining,
and take the images.
Good luck!
[Edited on 19-10-2009 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
qton
Harmless

Posts: 13
Registered: 19-9-2009
Member Is Offline
Mood: No Mood
|
|
Thanks for your reply, chemoleo.
>Very nice! The first one - why do you think these are bacteria? It could equially be cell debris (membrane, protein, polysaccharide aggregated
mucus). Bacteria are normally very small regular blobs that float everywhere, of equal sizes.
I figured since there were bacteria in saliva, they must be there somewhere and this was the closest looking thing I could find. Is it possible the
bacteria are elsewhere on that slide and because they're few in number I can't recognize them. QED. The only way you can really recognize bacteria is
to culture them and when you see large numbers of identically shaped things in certain patterns you know you've got them. (I guess you would have to
be a *great* pathologist to be able to spot a single one?) What about those large threads?
> The remainder - very nice sample of epithelial cells - from your nostril.
Ah! Thanks!
> What cameraµscope did you use? Can't be so cheap with the camera attached?
I picked up this microscope with this camera adapter (which screws on to a Nikon Coolpix) new on eBay. Not sure how it holds up against to a high-end
name brand, but so-far so-good.
http://cgi.ebay.com.au/40-2000x-Compound-Medical-Vet-Lab-Tri...
http://cgi.ebay.com.au/Microscope-Camera-Adapter-for-NIKON-C...
> Best is if you can make the cells attach themselves to a hydrophobic surface (plastic instead of glass slides) so that they stick - then you
can incubate them with whatever you want, and wash it off after. I.e. take up your mucosal scrapings in PBS buffer with a bit of glucose, let them sit
on a plastic slide for a while (6 hours, overnight), wash off everything then and see if something stuck to the slide. If so, carry on with the
staining, and take the images. Good luck!
Thanks for the tip! I didn't realize I could do this. I was watching a bio video the other night and what you just said with PBS clicked.
To see bacteria, I take it I'll have to grow them in culture like that too - albeit with agar. Is there a cheaper substitute I can use then agar
(which doesn't seem to be readily available).
Thank you again chemoleo!
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by qton  | | To see bacteria, I take it I'll have to grow them in culture like that too - albeit with agar. Is there a cheaper substitute I can use then agar
(which doesn't seem to be readily available). |
Agar is readily available at Asian groceries. The package I
have is "Golden Lion Trade Mark" Agar Agar (it's typically reduplicated) from a Hong Kong company, made in Korea. It was USD 1.50 for 1.5 oz (42.5 g).
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
The stuff sold as plain agar is usually just plain agar, the solidifying agent. You need to add nutrients such as meat infustion to the agar to coax
the little buggers to grow.
Edmond Scientifics used to sell nutrient agar on their website, but I have never seen it in their catalog.
Their is a recipe for nutrient agar in the Book of Amateur Projects by C. L. Stong in the Science Madness Library. Other books can be found at
books.google.com by searching for microbiology or culture media.
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by entropy51  | | The stuff sold as plain agar is usually just plain agar, the solidifying agent. You need to add nutrients such as meat infustion to the agar to coax
the little buggers to grow. |
Yes, but there are just so many different media. There's what looks to be a good
list of culture media here. I've not tried any of them, personally.
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Yes, there are thousands of flavors of culture media, but only a few dozen are used routinely in a given field of microbiology, medical, food, or
industrial.
This guy lists "rabbit dung" agar, which is one I've never run across.
Nutrient agar is a good choice for the beginning amateur because it will support the growth of common environmental bacteria, which are accessible in
soil, water and so forth. On the other hand it will not support the growth of the truly dangerous pathogens like plague or tularemia, thus providing
a measure of safety for the beginner. Not to say that everything growing on it is safe, however, and bacterial cultures must always be treated with
respect.
Plus nutrient agar is one of the cheapest. Edmund Scientific's nutrient agar is not the cheapest you can find, but they do sell to anybody.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Qton - when I looked at mammalian cells last, bacteria were about 1/20th the size of the mammalian ones, if not less (don't nail me on that one, just
judging by eye memory!  ) )
So small. And this was contamination - and these bloody creatures were absolutely everywhere. Almost like sandgrains, uniform, everywhere.
There's no way you'd be able to identify a single bacterium. There's usually lots of debris about - cells die, release lots of things (vesicles,
compartments, aggregates) - and all look similar under the microscope (unless you've got specific high-tech stains) - so I'd be careful in making a
'bacterial' claim.
For bacteria, there are of course gram -ve and gram +ve stains - check wiki on that, and see if you can obtain them.
Nowhere near a fraction of all bacteria grow in agar - so if nothing comes forth it means nothing unfortunately (as to whether you had bacteria or
not).
Check DMEM Invitrogen for what a common mammalian cell growth medium contains.
Oh, and the large threads? I could just say, debris, mucus, whatever - but certainly not bacteria!
Good luck!
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
qton, you might want to check out Todar's Textbook of Bacteriology while you're waiting for your books to arrive.
After you've done some more reading, you might want to think about ordering some culture media in Petri dishes and a Gram stain kit from Home Science Tools. Unless you have PCR and similar advanced methods, the first step in the identification of bacteria is normally to grow
isolated colonies in culture and perform Gram stain. Usually many biochemical tests are needed after that, but those are the first steps.
|
|
|
qton
Harmless

Posts: 13
Registered: 19-9-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by watson.fawkes  | | Agar is readily available at Asian groceries. The package I have is "Golden Lion Trade Mark" Agar Agar (it's typically reduplicated) from a Hong Kong
company, made in Korea. It was USD 1.50 for 1.5 oz (42.5 g). |
Thanks very much for the tip. A Korean friend said there's something called Carrageenan. Is this agar (or a little different)? BTW he said (and wiki
confirms it) that it's a carcinogen - though perhaps the order of peanut butter. Thanks also for the link to http://www.disknet.com/indiana_biolab/b030.htm ; Think I might roll my own Nutrient Agar. Do I need to buy Peptone or would boiling beef itself be
good enough? BTW this store looks good (and they're local to me). They sell all sorts of microscopy supplies... stains and Petri dishes even.
Everything except for agar (What's with that?) http://www.proscitech.com/cataloguex/home.asp
Quote: Originally posted by chemoleo  |
There's no way you'd be able to identify a single bacterium. There's usually lots of debris about - cells die, release lots of things (vesicles,
compartments, aggregates) - and all look similar under the microscope (unless you've got specific high-tech stains). Oh, and the large threads? I
could just say, debris, mucus, whatever - but certainly not bacteria!
|
Thanks, chemoleo. That's a vital piece of information I was missing. I'd assumed they would be pretty easy to see and identify (individually!). As it
is I've had the microscope for a couple of months now and I'm yet to definitely see one. At one stage I figured maybe my microscope was too weak, but
when I saw Prescott's Microbiology (they give magnifications on their pictures) I realised something was wrong. Fixing the staining seemed to help (at
least I can see the debris now :-)
Quote: Originally posted by entropy51  | | qton, you might want to check out Todar's Textbook of Bacteriology. ... You might want to think about ordering some culture media in Petri dishes and
a Gram stain kit ... Unless you have PCR and similar advanced methods, the first step in the identification of bacteria is normally to grow isolated
colonies in culture and perform Gram stain. Usually many biochemical tests are needed after that, but those are the first steps.
|
Thanks very much for the info, entropy51. No; don't have access to PCR (though at least I know what it is! :-) I'll order those Petri dishes and
stains. What's a good bacteria to (try to) cultivate for a beginner? Also I'm guessing for an incubator I can use an incandescent light (I looked at
egg incubators but strangely they're $$$.) Thanks for the texbook link too. Fascinating little blighters; I can't wait to see them.
[Edited on 2009-10-22 by qton]
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
"A Korean friend said there's something called Carrageenan. Is this agar (or a little different)? BTW he said (and wiki confirms it) that it's a
carcinogen - though perhaps the order of peanut butter. Thanks also for the link to http://www.disknet.com/indiana_biolab/b030.htm ; Think I might roll my own Nutrient Agar. Do I need to buy Peptone or would boiling beef itself be
good enough?"
- Carageenan and agar-agar, although both coming from red algae, are different polymers. Carageenan doesn't make a very stiff gel, so I would think
it's not that useful for microbiological application (you could have just as well grew the buggers in broth).
As for your second query, yes, making beef broth from butcher scraps should be quite sufficient for a number of garden-variety buggers. 
With regards to cultivation suggestions, E. coli (not the pathogenic strains of course!) might be a start. In the past I would've recommended
S. marcescens as well but evidence is mounting that they weren't as docile as first thought. Too bad because colonies of it are a nice shade
of red...
sparky (~_~)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by qton  | | Thanks very much for the tip. A Korean friend said there's something called Carrageenan. Is this agar (or a little different)? |
As previously said, they're different. Both are hydrocolloids, substances that form a gel in contact with water. A large amount of
what's going on in molecular gastronomy is hydrocolloid manipulation. There's quite a good recipe collection with these materials. Any of them could be used as a bacterial growth medium, although some are a bit pricey. You don't need the
recipes for this purpose, but the first page of each section has a table of material properties for a particular hydrocolloid.
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Casein tryptone
I'm interested in making some casein tryptone for growing lactobacillus. Is this as easy as stirring milk with papain (available as meat tenderizer or
as a nutritional supplement) and letting the enzyme do its work? Or is there some pH manipulation that's necessary/really-useful?
If any of you are curious, I'm curious to debug my yogurt-making process, which after the fermentation typically ends up with a small layer of whey on
top with a thin (about 1 mm) layer of culture floating on top. I think this has something to do that I ordinarily use 2% milk, that it's somehow
incompletely homogenized, and that it's separating. There's also a temperature gradient in the fermentation pot, and I suspect that there might be a
strain that grows preferentially in the slightly-cooler top layer.
|
|
|
| Pages:
1
2 |