guangdianlou208
Harmless

Posts: 14
Registered: 25-2-2009
Member Is Offline
Mood: No Mood
|
|
Talk about DAHA&ENTA again
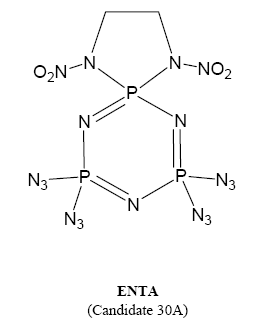
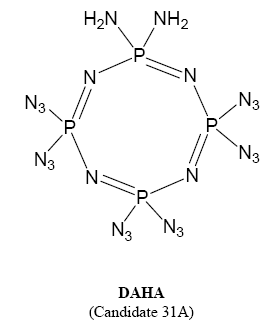
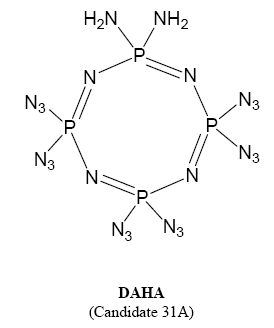
who can give some information about 1,1-diamino-3,3,5,5,7,7-hexaazidocyclotetraphosphazene(DAHA)
1,1-(N,N'-ethlenedinitramino)-3,3,5,5-tetraazidocyclotriphosohazene(ENTA).the two green primary expolsives.
thanks in advance!!!~~~
[Edited on 26-2-2009 by guangdianlou208]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Looks energetic. Hey, Axt!
Sic gorgeamus a los subjectatus nunc.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
That 6-membered ring with alternating trivalent N and pentavalent P atoms would be aromatic, - phosphazene - a rare example of non-organic
aromaticity, like borazine, B3N3H6, and its derivatives.
|
|
|
Axt
National Hazard
   
Posts: 821
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Same person that created the equally vague topic years ago?
I searched out the preps a long time ago yet it rests on the aquisition of PCl5. I believe the full preparations are in this mass of pdf's somewhere.
Vague question = vague answer (12.4MB).
http://rapidshare.com/files/202685231/Energetic_Cyclophospha...
[Edited on 26-2-2009 by Axt]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I believe Brauer has prep of the 6 membered P-N aromatic heterocycle, the other ring looks to be a relative of EDNA and the azide substitution would
be from the corresponding chloro compound. Indeed after downloading your zip file my supposition is born out. The starting material is from PCl5 and
NH4Cl.
Axt, the acquisition of PCl5 no longer rests on access to elemental P of any color. If you can acquite P2O5 and NaCl you can get to PCl5 by the
following route:
P2O5 -> POCl3 -> PCl5
The prep of POCl3: take your choice of reacting the pentoxide with oxalyl chloride (costly) or NaCl (cheap but best done in an autoclave).
With POCl3 in hand you can chlorinate to PCl5 directly or you can reduce to PCl3 with Sn and then chlorinate up to PCl5.
Another route is via PBr3, treat with Hg2Cl2 and filter off the Hg2Br2 or distill it off. That gets PCl3. Now chlorinate to PCl5.
Freedom from the tyranny of P.
These P-N heterocycles are analogous to cyanuric chloride and the azido derivatives to cyanuric triazide. Is the carbon analog of the EDNA adduct
known?
I attach the Brauer prep of the trimeric (PNCl2)3 which is isolated from the other oligomers by steam distillation, the others are all hydrolyzed.
This is the starting material for the diamino and diaminotetraazido derivatives including the EDNA functionalized one.
If you have PCl5 it is rather easy. Woelen take note.
[Edited on 26-2-2009 by Sauron]
Attachment: Pages from brauer_ocr-2.pdf (107kB)
This file has been downloaded 1018 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
guangdianlou208
Harmless

Posts: 14
Registered: 25-2-2009
Member Is Offline
Mood: No Mood
|
|
thanks a lot .the nitration of ENTA need nitronium tetrafluoroborate(NO2BF4),but NO2BF4 is very expensive, is there any other reagent
for nitration?
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
As far as I know that is the only one in the lit. Just like Picatinny Arsenal to use something as absurd as that. You know damned well that no
military explosive is ever going to be adopted that depends on such an expensive nitrating agent.
Then some other Dover NJ chemist will "discover" how to doit with HNO3 and get an attaboy from the Army. That's how those clowns operate, when they
aren't busy selling secrets to the Israelis, anyway.
(A retired engineer from Picatinny Arsenal was recently indicted for selling government secrets to the same Mossad handler who was implicated in the
case of convicted traitor Jonathon J.Pollard. The espionage took place in the 1980s, when the engineer was still employed by the Army. He is now in
his 80s. Why the FBI waited 20-odd years to make the arrest is as much a mystery as anything else.)
His name is Ben-Ami Kadish. Draw your own inferences from that.
http://www.nj.com/news/index.ssf/2008/04/expicatinny_enginee...
And like Pollard, he did it for $$$ and not out of some misguided loyalty to Israel. Treason foir cold hard cash. I hope they put him in a cold hard
cell.
[Edited on 27-2-2009 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Returning to chemistry:
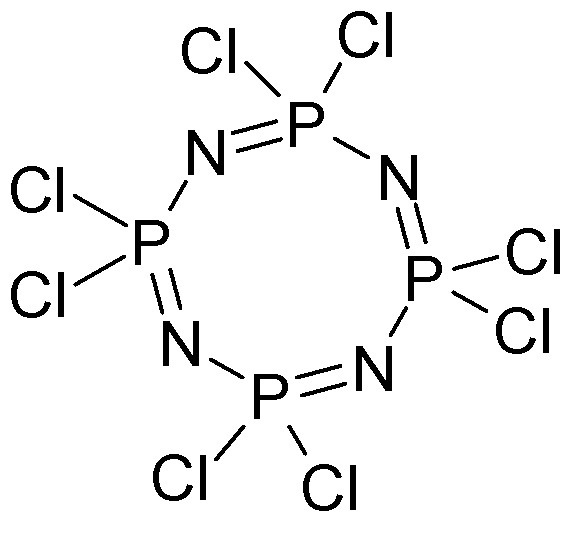
The phosphorylnitrilo chlorides are perfectly analogous to cyanogen chloride and like cyanogen they form cyclic oligomers.
3 NC-Cl -> (-N=C(Cl)-)3
n NP(Cl2) -> (-NP(Cl2)-)n n = 3,4,5,6, 7)
The N-C bond and N-P bond is the monomers is a triple bond
Where n > 4 the oligomers are oils or rubbers.
The n=3 compound is the 6-membered ring and is steam distillable, and crystalline once purified.
The DAHA explosive is derived from the n=4 heterocycle.
Two of the 8 chlorines are replaced with amino groups (on same P) and the other six with azido groups by reaction with NaN3 in acetone or
acetone-water.
These explosives are intended to replace lead azide in medium caliber cannon ammunition applications (primaries for electric or stab primers).
Personally I find these heterocycles intereting for themselves rather than for this rather far fetched "green explosive" program.
[Edited on 27-2-2009 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
guangdianlou208
Harmless

Posts: 14
Registered: 25-2-2009
Member Is Offline
Mood: No Mood
|
|
DAHA(1,1-diamino-3,3,5,5,7,7-hexaazidocyclotetraphosphazene)is new "green explosive",it's"lead-free", though aminating and aziding,(NPCL2)4's "Cl"
atom replaced by amido and azido.DAHA's melting point is about 75℃,it's not fit the demand of charge.
basic materil (NPCL2)4 is outgrowth of making (NPCL2)3,I cann't separate (NPCL2)4 from reacted slution.this is the real problem bothering me
[Edited on 28-2-2009 by guangdianlou208]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Please read the Brauer extracted pages I posted. There are differential solubilities that allow the separation from the n>4 oligomers and also from
n+3.
These are also discussed in the zip file posted by Axt
I do not understand what is the push behind lead free primers for medium cal (20-40mm) cannon. The Army and the AF do not use these in enclosed
spaces, and so maybe only the Navy has a need. We have been using lead azide and lead styphnate for many decades since they replaced mercury
fulminate.
Anyway what is wrong with cyanuric triazide? It is lead free. I sense the moinkees at Picaninny Arsenal just trying to justify their jobs as usual.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Microtek
National Hazard
   
Posts: 874
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Examination of firing range personnel has shown highly elevated levels of heavy metals in their bodies. That is one of the reasons that there is a
desire for heavy metal-free primers for small arms (there are also environmental concerns, but I don't know if those are involved in policy making).
As you say, this is hardly relevant for 20-40 mm munitions, but maybe logistical considerations can explain that...
As for LA and LS being used for a long time, that is true. However, many things which have seen extensive use have since been banned or restricted due
to health or environmental issues which were only discovered later. Examples include DDT (the insecticide) or atmospheric nuclear weapons testing. All
you can really do is to stop when you find out how bad it is...
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
There is a lead problem with firing ranges for handguns where lead bullets are used in practice ammunition because those are cheaper than full metal
jacketed ammunition. Typically we are talking about reloads with cast lead-antimony alloys used in projectiles. The contribution of the primer
composition is trivial.
So, that is a bogus issue.
The miltary lead replacement program is specifically medium caliber according to the documents in Axt's zip folder. And medium caliber is 20-40mm by
definition with small caliber ending at .50 (12.7mm) and anything over 40mm being large caliber. Those go up to 203mm histirically and for the Navy,
twice that. In contemporary terms 155mm is about it.
Sic gorgeamus a los subjectatus nunc.
|
|
|
guangdianlou208
Harmless

Posts: 14
Registered: 25-2-2009
Member Is Offline
Mood: No Mood
|
|
I didn't find the pages extracted by Brauer,which talking about separation of n=3 compound and n=4compound
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
It's not in axt's zip file, I posted it seperately, look upthread.
If you can't find it go to the forum library and download Brauser's A HANDBOOK OF PREPARATIVE INORGANIC CHEMISTRY and look in Phosphorus section.
Sic gorgeamus a los subjectatus nunc.
|
|
|
guangdianlou208
Harmless

Posts: 14
Registered: 25-2-2009
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Sauron
It's not in axt's zip file, I posted it seperately, look upthread.
If you can't find it go to the forum library and download Brauser's A HANDBOOK OF PREPARATIVE INORGANIC CHEMISTRY and look in Phosphorus section.
|
there is an reference named 《A Modified Method for preparation of a pure chlorocyclophosphazene tetramer》appered in《 Phosphorus
,Sulfur,and Sillicon》.the prepatation of octachlorocyclotetraphosphazene was gived.could you help me find this article,I use
octachlorocyclotetraphosphazene to made my green primer.
eagering!!!
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Give me the full citation not just title and journal
Authors
Volume and page numbers and year
DOI number
No DOI no paper.
and I will request the paper for you from References
Where is this reference given? In what paper or patent?
Post it here?
The DOI number can be obtained from the journal website.
Sic gorgeamus a los subjectatus nunc.
|
|
|
guangdianlou208
Harmless

Posts: 14
Registered: 25-2-2009
Member Is Offline
Mood: No Mood
|
|
I substituted two chlorine atom in (NPCl2)3 with ethylenediamine,the other four Cl will be substituted by —N3.
Utilizing sodium azide react with 1,1-spiro(ethylenediamino)-3,3,5,5-tetrachlorocyclo-triphosphazene in actone,
which is difficult to complete aziding.presumably due to NaN3 not disslove in actone. who can tell me how to
complete azido.
|
|
|
guangdianlou208
Harmless

Posts: 14
Registered: 25-2-2009
Member Is Offline
Mood: No Mood
|
|
TOP.
|
|
|
User
Hazard to Others
  
Posts: 339
Registered: 7-11-2008
Location: Earth
Member Is Offline
Mood: Passionate
|
|
WTF are you bumping this topic for this reason.
Sometimes a little more banning would be nice.
What a fine day for chemistry this is.
|
|
|