| Pages:
1
2 |
Methyl.Magic
Hazard to Others
  
Posts: 139
Registered: 14-5-2007
Member Is Offline
Mood: No Mood
|
|
Looking for Oxalyl Chloride (Swern ox) and 3-bromoanisole
Hello
I'm lookig for these two product.
Do you know if any chemical company (who accept to deliver at home not only to companies) has these products in stock ?
I live in Switzerland.
Thanks In Advance.
Methyl.Magic
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
Oxalyl Chloride is T+, hence you need someone with a license to sell it to you. It's not very likely that you could easily get it.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
That term is meaningless outside the EU (T+)
So it depends on where Methyl Magic is.
Anyway oxalyl chloride is not hard to make.
You need TCT (cyanuric chloride) and anhydrous oxalic acid to make it.
Or PCl5, but that is hard to get.
TCT is better. Either way expect a 50% yield.
Use a fule hood, oxalyl chloride is very irritating.
You can make your own TCT from methyl thiocyanate via chlorination. I have done threads on this before, UTFSE.
Can't buy methyl thiocyanate? Make it! Start with CS2. Again UTFSE.
Can't buy anything on this post? Move to a free country. I buy oxalyl chloride from Belgium.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Methyl.Magic
Hazard to Others
  
Posts: 139
Registered: 14-5-2007
Member Is Offline
Mood: No Mood
|
|
thank you Sauron and s_steve !
Yes I have my licence and I'm authorised to have toxic chemical in my possession but the problem is that in my country chemical vendors deliver only
to companies...
Sauron, you suggest to synth oxalyl chloride folowing this way :
CS2 -> methyl thiocyanate -> TCT -> oxalyl chloride
I thing that represent too much work for an available chemical... And CS2 isn't really easier to get than oxalyl chlorid itself...
You said you get it from Belgium, can you tell me your source ?
thank you !
|
|
|
harrydrez
Harmless

Posts: 26
Registered: 28-11-2008
Location: usa
Member Is Offline
Mood: content
|
|
He said that you can make your own TCT using methyl thiocynate, and you can make methyl thiocynate starting with CS2. However, TCT is what they use to
chlorinate pools and spas. It comes in those tablets and it's fairly available.
You would then use the TCT to chlorinate oxalyl anhydride.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Acros Organics
www.acros.com
It is not cheap! About $350 US a Kg.
The short synthesis of oxalyl chloride is with lots of PCl5. It is not very efficient, 50% yield. If you can buy red P in your country you can
generate Cl2 and easily make PCl5. The reaction with anhydrous oxalic acid is a solid phase one and is a great way to make POCl3, but a medicre way
to make (COCl)2. If you look at the mass balance you will see what I mean.
Or just buy cyanuric chloride (TCT) and make it with that. Yield is same as with PCl5. But this one is done in acetone with TEA as promoter, you gets
lots of precipitat and extract the oxalyl chloride from that with CCl4.
There are no other methods. So you have three choices.
Buy it
Make it by one method above or the other.
Go without it.
I suppose you also have the option I mentioned upthread. Move to a free country.
[Edited on 28-1-2009 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
| Quote: | Originally posted by harrydrez
He said that you can make your own TCT using methyl thiocynate, and you can make methyl thiocynate starting with CS2. However, TCT is what they use to
chlorinate pools and spas. It comes in those tablets and it's fairly available.
You would then use the TCT to chlorinate oxalyl anhydride. |
Where to begin? So much ignorance, so little time.
TCT is cyanuric chloride and is NOT used to chlorinate pools.
TCCA is trichloroisocyanuric acid and is what you are thinking of.
TCT is the acid chloride of cyanuric acid. The Cl replaces -OH groups linked to carbons
TCCA is a chloroamide, Cl is on N not C.
TOTALLY different compounds. Think before you post and make yourself look stupid.
TCCA cannot chlorinate anhydrous oxalic acid (there is NO SUCH THING as oxalic anhydride) Only PCl5 and cyanuric chloride (trichloro-s-triazine) can
do that.
The difference between TCT and TCCA and the confusion arising from the acronyms has been discussed and clarified MANY TIMES on this forum.
Read more and post less gibberish, fella.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The stoichiometry of the reaction between oxalic acid and PCl5 is
(COOH)2 + 2 PCl5 -> 2 POCl3 + (COCl)2 + 2 HCl (g)
The yield is 50% so you need 4 mols PCl5 to get one mol oxalyl chloride in practice.
Now look up the MW of PCl5.
You ought to be able to do it in your head.
One mol (COCl)2 is 126 g but requires almosy 833 g PCl5 to make it.
THIS SUCKS. To prepare a Kg you would need 7 Kg PCl5.
PCl5 is relatively cheap but the govt will be unhappy to see you buy it as it is a CW weapon precursor (nerve gases).
It is relatively simple to make from red P but the govt will be equally unhappy to see you buy that for same reason plus the meth cook application as
well.
So now you can see the logic of my alternative.
[Edited on 28-1-2009 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
biotech7
Harmless

Posts: 12
Registered: 27-1-2009
Location: Suzhou City, China
Member Is Offline
Mood: No Mood
|
|
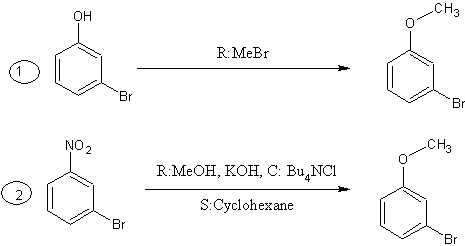
3-bromoanisole is slightly easy to be synthesized starting from 3-bromophenol or 1-bromo-3-nitrobenzene.
attachment is a hint for syn. route.
enjoy! 
[Edited on 29-1-2009 by biotech7]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Are you in the right thread, fella?
This one is about oxalyl chloride.
Sic gorgeamus a los subjectatus nunc.
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
Well, judging from the title, MM's asking about m-bromoanisole too, so...
sparky (~_~)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
biotech7
Harmless

Posts: 12
Registered: 27-1-2009
Location: Suzhou City, China
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Sauron
Are you in the right thread, fella?
This one is about oxalyl chloride. |
oxalyl chloride is irritant and dangerous if decomposed( a release of carbonyl chloride at high temp.). besides, it is probably hard to buy it
from chemical dealers or manuf. due to high transportion risk if your inquiry at small quantity
but syn. of oxalyl chloride is absolutely convienent in acceptable yields. 3-bromoanisole(also called m-bromoanisole) might be synthesized in
no more than 3 steps if you find it hard to buy it.
BTW, 3-bromoanisole is reasonably cheap and easy to buy outside your country.
B.R.
[Edited on 29-1-2009 by biotech7]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Methyl Magic, did you try contacting Swiss chemical companies like Fluka?
They belong to Sigma-Aldrich but they are in Switzerland so no EU bullshit to deal with.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Swern Oxidation: You can do the swern directly with trichlorotriazine (pool chlorinator), just do a literature search for this. I have not tried it
myself, but one could try to make the chloro-DMS intermediate quickly, and react any alcohol to see if you get the stinky DMS. Don't forget
triethylamine in step 2.
[Edited on 20-2-2009 by Arrhenius]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I am really getting tired of having to repeat myself but let's try one more time:
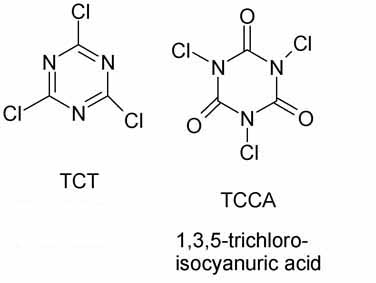
Trichloro-s-triazine, also called cyanuric chlorice or CC or TCT, is NOT a swimming pool cleaner.
The swimming pool cleaner is trichloroisocyanuric acid also called TCCA
These two compounds, while both derived from cyanuric acid, are structurally and chemically different.
TCT has chlorines on carbon
TCCA has chlorines on nitrogen
TCT is the acid chloride of cyanuric acid while TCCA is the chloroamide of isocyanuric acid
cyanuric and isocyanuric acid are tautomers.
I have posted drawings, and CAS numbers. You cannot use these reagents interchangeably.
Attempting to do so can be disastrous..
TCT is made industrially by combining hydrogen cyanide and chlorine to obtain cyanogen chloride which trimerizes to TCT.
In the lab it can be prepared by chlorinating CA with PCl5 or by chlorinating MeSCN with Cl2.
TCCA is made by chlorinating CA with Cl2.
CA is cyanuric acid.
PLEASE do not confuse these two different reagents again.
[Edited on 21-2-2009 by Sauron]
[Edited on 21-2-2009 by Sauron]
[Edited on 21-2-2009 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
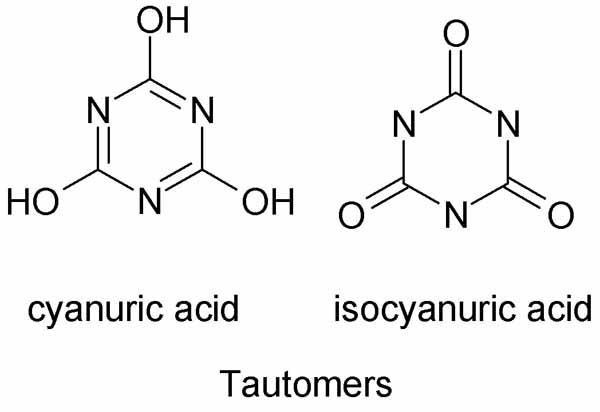
Also notice that while TCCA contains three carbonyl oxygens, TCT does not. TCT contains no oxygen.
TCT is a chlorinating agent
TCCA is primarily an oxidizing agent with some utility as a chlorinating agent as well.
They derive from the two tautomers of cyanuric acid.
TCT from cyanuric acid
TCCA from isocyanuric acid.
See structures below.
Only TCCA is a pool chemical. Put TCT in your pool and no one will ever show up to swim again.
[Edited on 22-2-2009 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
OK, fine. Buy some cyanuric chloride and run your Swern. Maybe you should make your own website where you're God, Sauron. Then you can rant and rave
all you want and not annoy people here.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The attempt to correct stupid mistakes of the ignorant is neither ranting nor raving. I said nothing about Swern oxidations. I said that
trichlorotriazine is NOT swimming pool cleaner, and you know what? I am right.
You want to wallow in misinformation, be my guest. Just don't dissminate it on this forum and expect others to go for it like hogs at a trough.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
Sauron was right and he didn't even take you personally to task until you insulted him. Be glad that you didn't learn the difference between TCT and
TCCA from personal experience.
PGP Key and corresponding e-mail address
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
I'm not denying your commentary, in fact I learned something. My point is, you post on these forums like a know-it-all rather than a teacher, and I
find that insulting.
I tried reacting some TCCA with DMSO anyway, and yes it reacts. Do I have a dry ice bath to do attempt a Swern with it? No. Do I have any idea
what's happening? No. Do you get DMS? Yes.
YOU, Sauron, suggested making oxalyl chloride from TCT. If one needs to buy TCT to get there, why not do the Swern with the TCT since this is now a
known reation? (will post references if you'd like)
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Sauron said nothing against using the TCT in the Swern oxidation. In fact he said nothing about the swern oxidation at all (other than mentioning he
didnt comment on it!). You should read his posts more carefully.
[Edited on 22-2-2009 by DJF90]
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Zzzzzzzzzz.... no, but he suggested making oxalyl chloride from it. Would be prudent to save yourself an entire step.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Read the thread title. LOOKING FOR OXALYL CHLORIDE
So don't knock me for telling the guy the slickest way to make oxalyl chloride.
"Swern Oxidation: You can do the swern directly with trichlorotriazine (pool chlorinator), just do a literature search for this. I have not tried it
myself, but one could try to make the chloro-DMS intermediate quickly, and react any alcohol to see if you get the stinky DMS. Don't forget
triethylamine in step 2."
[Edited on 20-2-2009 by Arrhenius]
You offered no literature to back up your assertion that TCCA can be used in the Swern, if that is in fact what you meant. If TCCA then TCT is not
relevent except for making oxalyl chloride. Lacking references or citations, and with some confusion as to which reagent you mean, we are left with
only your say-so, aren't we? Rather thin ice. The onus is on you to provide the lit.support.
If you really meant TCT, and it works as well as oxalyl chloride does, then yes, he could save himself a step. But that's a couple of big Ifs.
By the way, are we talking about chloromethyl methyl sulfide? Or sym. dichloromethyl sulfide? (Cl-CH2-S-CH2-Cl?
If so watch out because that is a deadly vesicant, carcinogen and homolog of mustard gas. It crosslinks DNA across the helix strands and prevents DNA
replication and repair. It's not merely stinky.
[Edited on 23-2-2009 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
I corrected myself on TCCA v. TCT, please see above post.
| Quote: |
If one needs to buy TCT to get there, why not do the Swern with the TCT since this is now a known reation?
|
huh? no chloromethyl.... put the chlorine on the sulfur (what I called chloro-DMS). Check the reaction mechanism for Swern Oxidation. Dimethylsulfide
(DMS) would be the break down product of the chloro dimethylsulfide intermediate in the Swern oxidation if you attempt to run it at 0ºC. DMS would
also be what is liberated when you decompose the formed salt by addition of triethylamine.
Here is a review article mentioning TCT (not TCCA) use in Swern Oxidations:
Current Organic Chemistry, 2004, 8, 1497-1519
See Scheme 34.
Here would be the JOC article cited:
De Luca, L.; Giacomelli, G.; Porcheddu, A. J. Org. Chem. 2001,
66, 7907.
[Edited on 22-2-2009 by Arrhenius]
[Edited on 22-2-2009 by Arrhenius]
[Edited on 22-2-2009 by Arrhenius]
[Edited on 22-2-2009 by Arrhenius]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Good old Lydia deLuca, grand dame of the TCT organickers.
She also did the TCT-DMF complex. Very handy.
Glad to hear "chloro-DMS} is not the chloromethyl. The proper name would be dimethylsulfenyl chloride.
(Me)2SCl
Sic gorgeamus a los subjectatus nunc.
|
|
|
| Pages:
1
2 |