| Pages:
1
..
13
14
15
16
17
..
28 |
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
| Quote: | Originally posted by Picric-A
Would it be possible to nitrate Benzoic acid and if so, what percentage of the Nitro group will go to the fourth carbon?
My aim is 4-nitrobenzoic acid by the way 
|
After nitrating benzoic acid under harsh conditions, you'll get mostly the meta-isomer, and only a touch of the ortho and para
isomers. That is because the carboxyl group is an electron-withdrawing, meta-directing group.
If it is the para isomer you wish, just oxidize para-nitrotoluene.
| Quote: | Originally posted by kclo4
sparkgap, thanks for the link but it seems to be oxidizing it further then I want.
|
I know, that's why I gave the link to the paper. 
I did find a JOC reference that claims to be able to turn amino acids to alpha-nitro acids... the catch is that you need hypofluorite in acetonitrile.

| Quote: | Originally posted by kclo4
Also, is something like Nitroacetic acid/nitroacetate easier to decarboxylate compared to acetic acid/acetate? |
It should. A nitro group attached to the alpha carbon of the acid has the same effect as the non-carboxyl carbonyl in acetoacetic acid.
sparky (~_~)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Picric-A
...
Would it be possible to nitrate Benzoic acid and if so, what percentage of the Nitro group will go to the fourth carbon?
... |
Very little, nitration of benzoic acid is used to make 3-nitro aand 3,5-dinitro benzoic acid; it needs concentrated acids aand forcing conditions.
Best to mono-nitrate toluene, isolate the p-nitrotoluene, and oxidise that to the benzoic acid.
But if you insist on starting from PABA, see
http://www.orgsyn.org/orgsyn/orgsyn/prep?prep=cv6p0803
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv3p0334
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv5p0845
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv5p0367
Note that while the oxidation with KMnO4 can give excellent yields, it only work well if at all with tertiary amines R3C-NH2
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by sparkgap
I did find a JOC reference that claims to be able to turn amino acids to alpha-nitro acids... the catch is that you need hypofluorite in acetonitrile.

sparky (~_~) |
*sigh*
haha but thank you  I'll look around some more, that was helpful I'll look around some more, that was helpful
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Picric-AMy aim is 4-nitrobenzoic acid by the way 
|
Here is a nice method for you Mr Picric Acid starting with PABA and oxidizing with DMDO which may be generated in-situ from oxone and acetone. 95%
isolated yield too! You may need to look up some other papers for preparation of DMDO solution but references are given within.
Attachment: Oxidation of Aromatic Amines with DMDO.pdf (919kB)
This file has been downloaded 868 times
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
And here is another very interesting paper for you Mr Picric Acid.
I go back to Taiwan in the new year for national service so maybe i won't be able to visit so much for a while. All the very best to you all for the
new year and keep doing interesting Chemistry! I have learned very much here and also with my studies. And now I have many interesting books too!!! I
finally realized that Nicodem was right, reading books is better than asking many questions! Thank you for all your help over past years and pray for
me that China sends more pandas and no bombs 
Attachment: A Mild Oxidation of Aromatic Amines with Oxone.pdf (114kB)
This file has been downloaded 10458 times
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
I will merely hasten to add that you should be very cautious in the handling of DMDO... it still is a peroxide of acetone after all. 
(edit: Alright, I will have to ask something after all. I am far away from my Merck Index, so could someone be so kind as to post the whole Merck
Index entry for the antihyperlipidemic acipimox? I am especially looking for synthesis-related references.)
sparky (~_~)
[Edited on 30-12-2008 by sparkgap]
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
crazyboy
Hazard to Others
  
Posts: 436
Registered: 31-1-2008
Member Is Offline
Mood: Marginally insane
|
|
Any idea how to synthesize tetramethylammonium nitrate? I don't really have much info other then its name.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
React tetramethylammonium chloride/bromide/iodide with AgNO3 in solution, filter off the silver halide ppt.
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
Surely as straightforward as tetramethylammonium hydroxide and nitric acid, right?
(edit: cripes, n_i gave the better one...  ) )
sparky (~_~)
[Edited on 31-12-2008 by sparkgap]
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Or react ammonia or (mono-, di-, tri-)methylamines with excess methyl nitrate?
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
SelfInflicted
Harmless

Posts: 15
Registered: 27-12-2008
Location: North America
Member Is Offline
Mood: ?
|
|
Today I wanted to make zinc chloride.
I dissolved some Zn metal, from zinc carbon batteries, and a scored copper penny in HCl from the hardware store sold as muriatic acid.
I put this on the hotplate to speed the reaction.
I was left with an oily solution that appears reddish brown with some black particles floating about.
What could cause this to appear reddish brown?
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
I believe Muriatic acid often contains some iron compounds in it.
Also, you didn't do this in a metal pot or can, and you did take out the copper left behind from the penny, correct?
I am going to guess it is probably an Iron or Copper impurity but is hard to tell until you give us a bit more detail.
By the way, You won't be able to produce dry (anhydrous) zinc chloride. ZnCl2 is a Lewis acid and you will either get a hydrate or an oxychloride.
|
|
|
SelfInflicted
Harmless

Posts: 15
Registered: 27-12-2008
Location: North America
Member Is Offline
Mood: ?
|
|
kclo4, thanks for the reply.
I am sure the muriatic acid I am using has some iron contamination. I would not think enough to have this as dark as it is. I am thinking the zinc was
not that pure and possibly the zinc in the penny is not as pure as most claims I have seen. Could be small copper particles also.
I tried another route.
This time I added zinc oxide in the HCl untill it would not react any more. This reation was exothermic.
I decanted the solution and left the un-reacted/undissolved zinc oxide behind.
I heated the solution to boiling and monitored the temperature at 133C there was not enough depth to accuratly measure with my PI thermometer. At this
point the solution was clear but slightly yellow, as is the muriatic acid.
I let this cool. It was not a reddish brown as the previous attempt. So I thought I would push this further to see what color I would get. I got a
salt forming at 203C (IR therm) and dry at 227C. Its very slightly gray/brown, i would say dingy.
So this must be Zinc oxychloride with some iron and other contanimants ?
It is hydroscopic and feels greasy and then oily.
I have tried to search the net for zinc oxychloride and can not find much about its properties. Any hints ?
I would also like to find out at what point does the zinc chloride convert to oxychloride and how.
I guess thats not such a short question thread.
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
Upon drying it, did it let off HCl fumes?
You either have Zinc oxychloride, or hydrated Zinc Chloride. At 227*C you probably have the oxychloride, but if absolutely no HCl fumes were let off,
maybe not.
Zinc chloride just converts to Hydrogen chloride and Zinc oxychlorides by hydrolysis. aluminum chloride, a much stronger Lewis acid, can be simply
heated with water and precipitate out aluminum hydroxide and produce hydrochloric acid (always thought this might be an interesting way to produce
hydrogen, since you are only using up water and aluminum while recycling HCl)
I found this interesting when reading about since chloride, How the heck does it work?
"Concentrated aqueous solutions of zinc chloride have the interesting property of dissolving starch, silk and cellulose, so that solutions cannot be
filtered through standard filter papers." - http://www.experiencefestival.com/a/Zinc_chloride/id/1952449
I can't find much on Zinc oxychlorides.. but I didn't look very hard.. perhaps there is a better name for them?
I think its chemical formula is Zn(OH)Cl, but I could be wrong.
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
My question is about cyanuric acid.
I've read in several places that it crystallizes from water as the dihydrate, but does anyone know how stable this hydrate is? For example, will
storing over H2SO4 in a dessicator leave the anhydrous product, or will it not lose any water? I've been meaning to do some experiments, but the
stoichiometry will go out the window if I don't know if it's hydrated or not. Thanks.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
jhullaert
Harmless

Posts: 13
Registered: 11-12-2008
Location: Ghent Belgium
Member Is Offline
Mood: No Mood
|
|
Hi
I have a small problem and I thought this was the best place to ask it.
I want to know the pKa value of caffeine.
When I searched on google I found every value starting from -1.14 to 50.
First I have to know if caffeine is an acid. I've read that a 1% solution has a pH of 6.9 so it has to be a weak acid if it's correct. Maybe they
found this value using a digital pH-meter that gave a slightly wrong measurement.
If it's an acid then it's probably the methylated amine group (between the two carbonyls that loses a proton. The charge is divided between the
methylated amine and the 2 carbonylgroups. Is this correct?
thanks
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
No. The nitrogen between the carbonyls has formed 3 bounds, none with a plain hydrogen (no N-H). You are right a compoud with a nitrogen between two
carbonyls can act as an acid, like in the case of phthalimide. But a N-H bond is required, and this nitrogen is methylated.
All other nitrogen can be protonated as far as I know, but it doesn't seem to be very likely that that occurs to any great extent, looking at the
structure, especially not with something like water (strong acids like HCl should do the job though).
There are 10 hydrogens in caffeine, of wich 9 are in the methyl-groups. The final hydrogen is bonded to the carbon on the 5-ring, and this is the only
possible candidate. Normally C-H bonds will not break up giving a proton and carboanion, because of the great stability of a C-H bond and the high
instability of a carboanion.
However, this carbon is bonded to 2 nitrogen's, of wich one is a double bonded. Both these nitrogen's are electron withdrawing and pull the electrons
of the carbon towards the nitrogen. This has as a result that the electrons of the C-H bond are pulled more closely to the carbon, and therefore
making the C-H bond more loose/weaker. Therefore I think it would be likely that caffeine is SLIGHTLY acidic. However please note that at pH 6,9 the
H3O+ concentration is still EXTREMELY low.
I'm n ot sure if I'm right here, it's just my hypothesis. Can anyone tell me if this is true?
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
http://en.wikipedia.org/wiki/Caffeine#cite_note-0
The pKa values of -0.12->1.22 are for the *protonated* form. That means the protonated form is a rather strong acid and therefore the
non-protonated form is a weak *base*.
Of course that aromatic H is probably quite easily nibbled off, making the whole thing amphoteric.
|
|
|
jhullaert
Harmless

Posts: 13
Registered: 11-12-2008
Location: Ghent Belgium
Member Is Offline
Mood: No Mood
|
|
So the protonated form is with an hydrogen at the double bonded nitrogen atom of the 5 ring. is that correct?
thanks for helping me.
Jor how was your holiday?
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
I'm not sure wich N-atom is protonated, e.g. wich N-atom had the strongest basic properties. I do know that the hydrogen is at the C-atom in the
5-ring is the only hydrogen wich can be splitted of as a proton.
My holiday was great. Thailand. What a country! 
Rotterdam is just a village compared to the chaos in Bangkok.
Only one thing made me a bit scared. My dad and sister wanted to buy some Thai vegetables at the airport as a present, and took it in their hand
luggage. I told to do not, but they did. We had some problems with the customs. Gave me thrills, I know what happens when you smuggle something:
Bangkwang aka Bangkok Hilton. Ofcourse I knew we did nothing wrong, but when you're there, it's not something nice.
And yours? 
|
|
|
hector2000
Hazard to Others
  
Posts: 127
Registered: 22-8-2006
Member Is Offline
Mood: Cool
|
|
what is name of this componet?this componet is possible?if yes what is another name of it?

thx
Chemistry=Chem+ is+ Try
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by hector2000
what is name of this componet?this componet is possible?if yes what is another name |
acetophenone
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
That is the enolic form of acetophenone. Simple enols like this one are not stable compounds. They can only be prepared in completely neutral media
and their shelf life is generally less then 30 minutes. In the slightest presence of acids or bases they immediately tautomerize to the corresponding
ketone in matters of seconds.
|
|
|
jokull
National Hazard
   
Posts: 506
Registered: 22-2-2006
Location: Everywhere
Member Is Offline
Mood: Ice glassed
|
|
| Quote: | Originally posted by hector2000
what is name of this componet?this componet is possible?if yes what is another name of it?

thx |
This is called 1-phenylethenol. It should be possible since it has a CAS number: 4383-15-7.
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
It's possible to trap that form by making the OH group an ether. Trimethylsilyl is fairly commonly seen, I believe. You'd need trimethylsilyl
chloride and a strong base to generate the enolate.
[Edited on 1-8-09 by UnintentionalChaos]
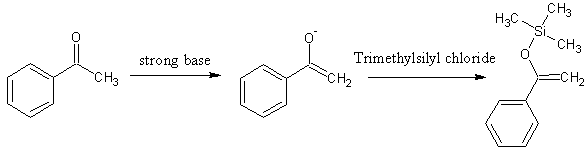
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
| Pages:
1
..
13
14
15
16
17
..
28 |