Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Chloratomercuraldehydes and relateds
The first is: Chloratodimercuraldehyde, OHC.C(:Hg).Hg.O.ClO2 = C2Hg2ClO4H, this one is best to avoid altogether. But for curiosity
sake: a yellow mercury oxide is added to a dilute chloric acid solution (made from Ba-chlorate) so that about half of the oxide remains undissolved.
Then to this basic chlorate, decanted mixture: add under ice cooling and under continuous shaking (hrmmm, those were the instructions) about so much
alcoholic aldehyde solution is added as approximates to the formula, and then allow the crystallization to go on in the cold. The crystals explode
with utter violence if shaken under the liquid (!), so they can only be taken out very carefully with a soft brush. If 0.2 g of the compound in the
free is: exposed to a weak shock, an electric spark, or contacted with a flame, it causes a bright, tremendous bang, and the material under it becomes
deformed like as if hit by a bullet.
Chloratotrimercuraldehyde ClO3.Hg(Hg2O):C.COH = C2Hg3ClO5H, this is less dangerous than the substance above, and is made easily by
passing acetylene into an aqueous mercuric chlorate solution, or a mixture of mercuric nitrate and sodium chlorate solution. It precipitates soon as a
white solid. Known to explode on contact with a flame or conc. H2SO4. Deflagration temp.: 130 deg.C. This has also initiating action, where 0.4 g is
the minimal needed for PA.
Perchloratotrimercuraldehyde. Although worse in the lead block test (18.3 cc vs. 25.6 cc per 2g, table below) it is a better
initiator than Hg(ONC)2, at least for picric acid, where 0.1 g is the minimal amount needed to initiate PA, compared to 0.0237 g for AgN3 and 0.25 g
for Hg(ONC)2. The compound may be more described in Ber. 38, 1999, 1905.
Sources:
Handbuch der angewandten physikalischen Chemie in Einzeldarstellungen by Georg Bredig, Wilhelm Bachmann. 1908. Vol. 9-10. Pg: 118. Table.
Jahresbericht über die Leistungen der chemischen Technologie by Johannes Rudolf. 1906. Jahrg. 51. Pg 461.
Beilstein 606-607 (Sys. No. 279).
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
The compound 2-methyl-1-nitratodimercurio-2-nitratomercuriopropane
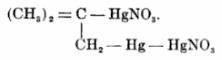 = C4H8Hg3N2O6 = C4H8Hg3N2O6
made from isobutylene and Hg(NO3)2, explodes from impact or heating to 80ºC. And HCl acid reforms isobutylene alongside forming mercuric and
mercurous chloride (Organic Compounds of Mercury by F.C.Whitmore (1921), p. 116).
The compound:
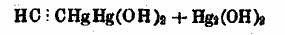 = C2Hg4O4H5 = C2Hg4O4H5
forms next to C2H.HgO + HgCl2, by prolonged boiling of the mercury compound of vinyl alcohol, CH2:CH.OHgO + Hg2Cl2, with KOH solution (Ber. 22,
2865-7), this is a black to dark-green precipitate which explodes extremely violently at 157ºC (0.1 g was heated on paraffin bath). The explosion
destroyed apparatuses and blew them to pieces. This compound could not be made to explode by shock.
The compound:
 = C2Hg6O4H2 = C2Hg6O4H2
also called a mercarbide, is made from yellow mercury oxide by boiling with aqueous-alcoholic KOH or NaOH, as done by Hofmann (Ber. 1900, 1328), who
could obtain the base as the end product of reaction of yellow HgO and some aqueous alkali and: EtOH, acetaldehyde, propanol, allyl alcohol, amyl
alcohol, cellulose, starch, and cane sugar. It is said to be extremely stable against aqueous compounds, is quite basic, and explodes extremely
violently at higher temperatures (230 deg.C.), but it is said to be stable to shock. It is said to be very stable against hot conc. acids and bases,
and strong oxidizers. Upon prolonged heating with strong HCl acid it forms the chloride, C2Hg6Cl6, etc. For more on this compound see Whitmore, pgs.
125-128, etc.
[Edited on 9-8-2008 by Schockwave]
|
|
|
Axt
National Hazard
   
Posts: 825
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
I've tried ethane hexamercarbide a few years ago now, though the failed attempt just bumped like crazy spreading mercuric crap around. It forms salts
with oxidising acids as well, nitrate decomposes, perchlorate deflagrates, bromate and chlorate explode vehemently (Ber. 38, 3654-59 (1905)).
More on its preparation:
When sodium (10 grams) is dissolved in alcohol (150 grams) and the solution boiled (16 hours) with finely-divided yellow mercuric oxide (40 grams),
the residue contains, in addition to mercury and mercury oxide, a new substance, yellow in colour but turning grey when exposed to light, which
appears to have the constitution OHg2:C(Hg.OH).C(Hg.OH):OHg2, and is a member of a class of compounds termed by the author oxymercarbides. When
boiled with an aqueous solution of hydrazine, it yields nitrogen mixed with some ethane. It explodes violently at about 230, but is not very
sensitive to percussion. It is a diacid base, forming insoluble salts from which it is regenerated by treatment with alkalis; the nitrate sulphate,
and chloride appear to have the constitution C2(Hg.NO3)2(Hg.OH)4, C2(Hg.SO4H)2(Hg.OH)4, and C2(HgCl)6 respectively. So firmly are the carbon and
mercury united, that potassium cyanide does not separate them, but forms instead a yellow-compound, C2Hg2(HgCN)2. Paraldehyde yields the same
mercarbide, and acetone and propylic alcohol form analogous ones. Methylic alcohol, however, does not form a mercarbide. (Ber. 31, 1904-9 (1898)).
The compound, OHg2:C(Hg.OH).C(Hg.OH):OHg2, is proved to be a derivative of ethane. It is best prepared by the action of yellow mercuric oxide and
aqueous alkali on ethyl alcohol. The base is also obtained, although in smaller yield, if acetaldehyde, propyl alcohol, allyl alcohol, amyl alcohol,
cellulose, starch, or sucrose are used in place of ethyl alcohol. (Ber. 33, 1328-39 (1900)).
|
|
|
|