Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
Another caged nitramine
Hi all, recently i got some interesting information about compound shown on picture below. It's very powerfull exposive, even more then CL-20 and HMX.
Only drawback it is hydrolized by water on boiling and looses one nitrogroup.

Properties of compound: Melting point 205C (with decomp.) explodes at 210C. Crystall density 2.07 g/cm3, heat of explosion 5.84 megajoules/kg.
Sensitivity to impact is on HMX level 21cm with 2.5 kg weight (HMX - 26 cm). Detonation velocity 9800 m/sec, detonation pressure 46.1 GPa, throwing
ability is 118% of HMX (HNIW - 108%).
Compound can be made in completely referenced route starting from glyoxal and formamide. Using the following route:


Any oppinions?
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|

| Quote: | | Boyer and coworkers reported the synthesis of cis-syn-cis-2,6-dioxo-1,3,4,5,7,8-hexanitrodecahydro- 1H,5H-diimidazo[4,5-b:40,50-e]pyrazine (16) and
cissyn- cis-2,6-dioxo-1,4,7,8-tetranitrodecahydro-1H,5Hdiimidazo[ 4,5-b:40,50-e]pyrazine (17) by nitration of the parent dihydrochloride salt with 20%
N2O5/HNO3 or Ac2O/HNO3, respectively. Compound 16 which has a crystaldensity of 2.07 g/ml,the highestdensity recorded for a C, H, N, O explosive,
decomposes explosively at 210 8C and is decomposed easily with water. Compound 16 is probably the most powerful explosive synthesized to date.
|
http://enermat.org.ru/nitro.html
|
|
|
Axt
National Hazard
   
Posts: 825
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
I wouldn't call it a caged structure, tricyclic.
I attached this previously, J. Org. Chem. vol. 56, pp. 3413-3419 (1991).
http://www.sciencemadness.org/talk/viewthread.php?action=att...
If you do a search for HHTDD you'll pull up a bit more information.
Chinese Journal of Structural Chemistry, vol. 22, pp. 223-227 (2003) gives measured velocity of 9019m/s @ 1.862g/cm3, 9546 @ 1.995 and extrapolated to
9800 @ 2.07 (TMD).
I aquired the needed precursors; glyoxal, formamide and P2O5 though never went through with it.
Why do you say its hydrolised losing one nitro group? One would expect the two dinitrourea moieties to hydrolyse yielding 4 primary nitramine groups,
releasing CO2.
[Edited on 4-8-2008 by Axt]
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
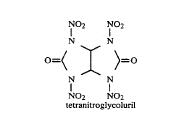
These compounds are very similar to tetranitroglycouril. The attached graphic comes from a 1984 SNPE patent: http://www.pat2pdf.org/patents/pat4487938.pdf. It too is made from glyoxal & formamide. It doesn't take much imagination to get from there to
the more elaborate structures you've shown.
[Edited on 3-8-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
Synth of this compound was successful, description can be found in this topic: http://www.sciencemadness.org/talk/viewthread.php?tid=5997
[Edited on 8-4-2009 by Engager]
|
|
|