Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
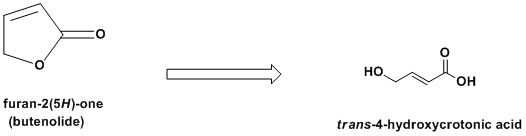
Furan-2(5H)-one & trans-4-hydroxycrotonic acid
In reading that Danish paper that I had posted earlier, it noted that conformationally-restricted analogs such as trans-4-hydroxycrotonic
acid (T-HCA) are more potent than GHB. I searched here for that compound & found nothing. I have been unable to check this key paper:
| Quote: | | Bourguignon et al (1993) Analogues of γ-hydroxybutyric acid. Synthesis and binding studies. J.Med.Chem. 31 893 |
If anyone has a pdf of this paper I'd appreciate their posting it.
I did find a simple synthesis of the immediate precursor of T-HCA: furan-2(5H)-one (butenolide). See http://www.orgsyn.org/orgsyn/pdfs/CV8P0396.pdf. It involves a pretty nasty oxidation of a cheap starting material: furfural.
[Edited on 6-7-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
leu
Hazard to Others
  
Posts: 368
Registered: 13-10-2005
Member Is Offline
Mood: No Mood
|
|
The requested paper:
Analogues of gamma-hydroxybutyric acid. Synthesis and binding studies.
Bourguignon JJ, Schoenfelder A, Schmitt M, Wermuth CG, Hechler V, Charlier B, Maitre M.
J Med Chem. 1988 May;31(5):893-7.
Substituted 4-hydroxybutyric (GHB) or trans-4-hydroxycrotonic acids (T-HCA) and structurally related compounds were synthesized and submitted to
[3H]GHB binding. Structure-activity relationships studies highlighted for [3H]GHB binding (a) the necessity of a nonlactonic, relatively extended
conformation of the gamma-hydroxybutyric chain, (b) the existence of some bulk tolerance in the vicinity of the hydroxyl group, and (c) the high
sensitivity toward isosteric replacements of the carboxyl or the hydroxyl groups. T-HCA has been recently identified as a naturally occurring
substance in the central nervous system (CNS) and shows a better affinity than GHB. Our findings are in favor of the presence in the CNS of specific
GHB binding sites, which are different from the GABA and the picrotoxin binding sites, and for which T-HCA may be an endogenous ligand.

Attachment: Analogs_of_gamma_-hydroxybutyric_acid_Synthesis_and_binding_studies.pdf (679kB)
This file has been downloaded 977 times
Chemistry is our Covalent Bond
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by leu
The requested paper:
 |
Yes, I agree. And thanks for the reference.
Here is a summary of the JMC synthesis from the Rhodium WS:
| Quote: | trans-4-Hydroxy-Crotonic Acid (T-HCA) [13,19]
Comment: T-HCA is 16% more potent as a GHB receptor agonist than GHB itself, and most T-HCA (trans-4-hydroxy-2-butenoic acid) derivatives are more
active than the corresponding GHB homologs. The 4-CH3 analog is 9%, and the 4-Ph analog is 27% more potent than GHB itself. The 4-C6H11 analog is 16%
less potent than GHB, and cis-4-Hydroxy-Crotonic Acid (C-HCA) is inactive. T-HCA has also been identified as a naturally occurring substance in the
CNS, which dismisses the theory of T-HCA just being a synthetic semi-rigid analog of GHB, but as a possible endogenous receptor ligand, which also
competes at GHB receptors, and possibly possesses specific functions of its own.
Synthesis:
To a solution of 20g (0.23 mole) of crotonic acid (2-butenoic acid) in 200ml of dry benzene, 45.6g (0.25 mole) of N-bromosuccinimide was added under
nitrogen. The solution was brought to a gentle reflux with stirring and was treated with 0.5g (3.7 mmol) of 2,2'-azobisisobutyronitrile as a radical
initiator. Refluxing was continued for 2 h, and the solution was cooled to 10°C. The resulting white precipitate was filtered off, and the filtrate
was evaporated in vacuo. The residue was taken up with 200ml of carbon tetrachloride and the mixture was cooled to 0°C and filtered. The filtrate was
evaporated in vacuo to give 38 gram of a mixture consisting of 85% 4-bromocrotonic acid and the rest unreacted starting material. Pure 4-bromocrotonic
acid can be obtained by multiple recrystallizations from petroleum ether.
To a cold solution of 12g (72 mmol) of 4-bromocrotonic acid in 120 ml of water was added dropwise 240 ml of a 2M KOH solution in water. After the
addition was completed, the solution was successively heated under reflux for 5 minutes (oil bath temp 120°C), cooled in an ice bath, and acidified
with dilute H2SO4. The medium was evaporated under vacuum, and extracted with ethyl ether. After the drying and evaporation of the solvent, the
residue was chromatographed on a silica gel column eluted with a mixture of EtOAc:MeOH (97:3) to yield 5.22 g (71%) of pure T-HCA. After
recrystallization from EtOAc, the mp was found to be 108°C.
|
Sounds like a lot of work. As I've pointed out, there are other ways to get to T-HCA from more readily available starting materials than crotonic
acid, bromine & AIBN.
BTW, that pdf file downloads as a .php document but it opens if you select Adobe Acrobat.
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
ShadowWarrior4444
Hazard to Others
  
Posts: 226
Registered: 25-4-2008
Member Is Offline
Mood: Sunlight on a pure white wall.
|
|
| Quote: | Originally posted by Ritter
BTW, that pdf file downloads as a .php document but it opens if you select Adobe Acrobat. |
This can be a problem with many PDFs downloaded via the forum--the solution I use is to copy the name and extension of the document, and place them in
quotes when saving the file. This instructs windows to save it as a .pdf. I also change the bottom menu from .php to 'All Files,' just incase.
[Edited on 7-6-2008 by ShadowWarrior4444]
|
|
|
Methyl.Magic
Hazard to Others
  
Posts: 139
Registered: 14-5-2007
Member Is Offline
Mood: No Mood
|
|
| Quote: | Message original : Ritter
I did find a simple synthesis of the immediate precursor of T-HCA: furan-2(5H)-one (butenolide). See http://www.orgsyn.org/orgsyn/pdfs/CV8P0396.pdf. It involves a pretty nasty oxidation of a cheap starting material: furfural.
|
When you saponificate the crotonolactone you will get the cis-product not the trans one... no ?
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Methyl.Magic
| Quote: | Message original : Ritter
I did find a simple synthesis of the immediate precursor of T-HCA: furan-2(5H)-one (butenolide). See http://www.orgsyn.org/orgsyn/pdfs/CV8P0396.pdf. It involves a pretty nasty oxidation of a cheap starting material: furfural.
|
When you saponificate the crotonolactone you will get the cis-product not the trans one... no ? |
I believe the trans isomer is thermodynamically favored over the cis form. But since the cis form is biologically inactive, you would have a quick
qualitative test of which isomer you had.
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|