| Pages:
1
2 |
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Solvent Stills
I'm considering installing a solvent still at home. I recycle practically all my solvents, using a conventional fractionnal distn setup, after
treatment of the crude solvent (washing with either conc. H2SO4 or base, K2CO3, brine, etc) and reflux over a proper dessicant.
Mostly using 19/26 glassware, these distn are very-time consuming, and do not allow partial reflux, require stopping the heating after initial
reflux, attaching a dry fractionnal distn apparatus, and heating again, etc.
At work, we have turned to using continous solvent stills for each solvent, for those not familiar the solvent is constantly refluxed over a suitable
dessicant, under a stream of inert gas, and collected in solvent still heads (picture ), so fresh solvent is constantly been distilled into the head, and can be withdrawn with a syringe, or drained into a schlenk.
I have no need for such stills, as i only need performing batch distillation, after a prior reflux, either in a schlenk for rigorously dry solvents,
or in a simple receiver (no inert gas) for recycling regular-grade solvents (acetone, methanol, toluene, etc).
Now, at work, we used to have small stills under a hood, for batch distn of solvents. Since we now have designated rooms with a still for each
solvent, nobody uses them anymore, and I just can locate the old stills. I was looking for one to bring back home if unused and ok with my boss, or at
least show the model to the glassblower. I have looked all other the net for a picture or drawing on such a still, but not find any. I'm not refering
to a conventional reflux head, a bit like stoechiometric_steve's impressive still displayed in the "tour my lab" thread, but of a system half-way
between a perkin triangle and a dean stark trap, (poorly) represented here:

Once the tap under the reflux condenser is entirely closed, full reflux is set. If totally opened, direct distn occures. The reflux ratio can be set,
ie for 4 drops falling from the condenser, one falls in the receiver, the rest falls back into the still pot.
I would like to know if anyone here posses, or uses at work, this or a similar device, and if so where he bought it and for how much, to see if it's
worth asking for a custom glassware (which would be relatively expensive). I have checked aldrich and other companies catalogue, most of them only
have the recent (and f$*@ing expensive) reflux heads. The glassblower from which the lab used to buy/get repaired the glassware is now retired, so i
have no means of knowing if these were custom-made of commercially produced.. i could always ask the administration to check the bills, but that would
be a very long process, and it's not sure anything will be mentionned... I asked a few colleagues, most of them didn't have the slightest opinion, so
I figured I could ask here.
I also wanted to know what kind of setups people have installed at home, I have already seen Steve's beautifull still, and would be delighted to see
other setups.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
F2Chemist
Harmless

Posts: 28
Registered: 30-6-2008
Member Is Offline
Mood: No Mood
|
|
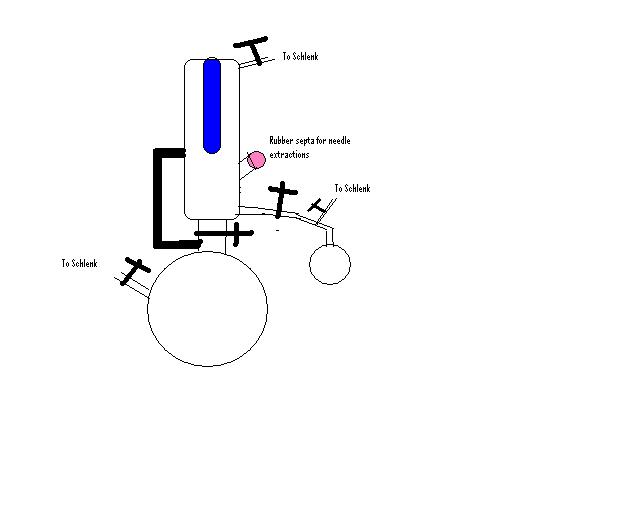
We use something similar, here's a very rough sketch.
The blue bit is the water circulator/cooler. Crosses are valves (they are quite expensive, but I prefer teflon valves) and the large black line on
the left is a large diameter glass tube.
During drying the valve above the pot is open, allowing easy reflux. After the solvent has been drying for a while we close that valve, allowing the
upper chamber to fill up until it reaches the level of the large diameter glass tube (the black line), where it then spills back down into the pot.
We then can take small amounts through the rubber septa or larger amounts into another round bottom.
Once dry, we usually are always refluxing the solvent, but keep the valve above the pot closed, this circulates the solvent in the upper part.
As always we keep the system under a slight positive nitrogen pressure.
Good luck
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Thank you for th ecomment. That system is pretty similar to the still head I linked too. Unfortunaly, this isn't what I'am seeking, as this sytem,
like those at work I described, make avalible a constantly renewed amount of solvent in the head, and is refluxed all day.
I will be performing batch distn, receiving the dried solvents in schlenks that will then be stored under Ar and 3A MS, after a few hs reflux over
adequate dessicant, or simply repacked into amber bottles for regular-grade solvents. At first, iw as thinking of using my Dean Stark, but agin this
is a 19/26, and I have no means of connecting a schlenk to the draining of the trap. I guess I could be using it for solvents I need in small
quantities and not rigorously anhydrous. After all, the system I described is very similar to a Dean Stark trap, except the the trap itself has an
insgnificant volume (<1mL).
I will show the diagram to my glassblower and see if he knows if these can still be bought commercially, and if not how much it would cost to have
one made in 29/32. I will surely place a vigreux between the still and the head, depending on the contamination of the recycled solvent and the purity
required. With an adequate reflux ratio, the theoretic nb of plates can be significantly increased IMHO. The neck of the head (connected to the pot)
could also be filled with steel wool or another garniture.
One major draw back is that it isn't possible to measure the vapor temp, no fitting for the thermometer. With pur solvents this isn't a problem, but
for recycling solvents containing traces of other more volatil compounds (I have, for example, several liters of AcOET/Pet ether used as eluant for
columns and distilled off the products with a vigreux, providing a very gross seperation. The pet ether-rich fraction will be washefd with conc. NaOH
no problem, but the AcOET will require carefull fractionnation. Ethyl acetate is very cheap (8E 5L), but I dispose of my waste at a chemical waste
company, which charges roughly 1E per liter... So I prefer keeping the amount of waste minimum.)
Well, I suppose that for general uses traces of hydrocarbon won't be problematic, and that if I need very pur AcOEt, I will start of from the
commercial product.
Sorry, started thinking out loud 
I'll post some pictures if/when I get it installed 
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Klute:
The reflux ratio valve I've used on several columns (from quite small to a 10 m pilot plant for rectification of methanol to UV grade) is based on a
timed valve. Basically a timer circuit determines the amount of time the condensate flows back into the column and the amount of time condensate is
tapped off as distillate. On the large column a timing cycle would be typically 40 s of complete reflux to 20 s of tapping off the distillate. Any
other reflux ratio can of course be obtained by changing the ratio of the valve position: the valve is essentially a two position valve
(open/shut) operated by a solenoid motor (on/off). On smaller columns the total time cycle would be shorter, typically 15 s or so...
In practice the column therefore operates in 100 % reflux a set amount of the time and in zero reflux for the remaining time of the cycle. In reality
this clearly approximates a preset reflux ratio. We distilled UV grade methanol from pretty mean technical methanol very successfully this way.
I don't have photos of this type of reflux ratio condenser but I'm sure a Google search would unearth some.
Interesting thread BTW.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
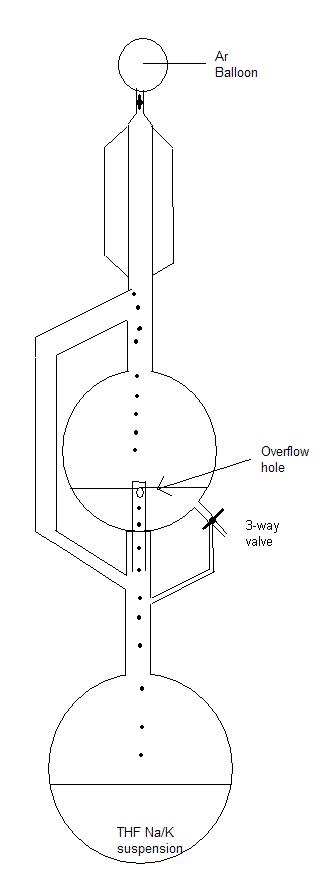
We have two continuous reflux stills for diethylether and THF over Na/K alloy. I have drawn a schematic which I hope is clear enough.
Basically THF vapor rises through the sidearm until it reaches the condensor, where it drips into the collection flask. This flask is equipped with a
3-way valve which can be used for taking THF from the flask or to flush it back into the heating flask (in cases where it has been standing overnight
without continous reflux for example).It also has a protrusion from beneath with a hole, through which THF flows back into the heating flask once it
reaches a certain level. Schlenk line is not necessary, flushing the apparatus before use and equipping it with an Ar balloon is sufficient. This will
supply you with dry THF that is suitable for anionic polymerizations.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Here's an interesting thread from a home distillers forum.
And this *.pdf describes very accurately (includes photo) a commercially available top-of-(glass) column timed reflux splitter, very close to the kind I've used myself and was describing above.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Thank you all. I remeber those electronic time splitters when working on semi-industrial rigs (continous rectification, and reaction mostly). again,
the reflux ratio was set by the ratio time open/time closed.
I really like the setups the hombrewers have made, very impressive.
I've realized I could actually use my Dean Start as a reflux splitter, attaching the trap exit into a thermometer adaptator on top of a schlenk. The
only disavantage is that th estopcock isn't veyr precise, so it will be fidly to correctly set a precise reflux ratio, and I'm not sure how
impermeable the teflon stopcock is to air. I will try to fit a glass one in (although this means a little grease in the distd solvent). On the stills
we had at work, there were screw-in stopcocks, have a much smaller possible-contact area, and been pretty much sealed (we ayhd the same for addition
funnels, and never caused any problems with very reactive reagents. Although once a colleague was adding BuLi with such a addition funnel, apparently
the srew wasn't correctly attached, as 5min after the beggining of the addition, the outside of the funnel spontanously caught fire: BuLui had be
acumulatiing around the screw and finished by igniting...). I will try it for small amount of dry solvents such as THF, but still need something else
for the other solvents..
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
IMO Chemglass makes the finest solvent stills sold. I really like their design. They have both the 2500ml and the 1000ml resevoir heads... my price is
around $280ml for the 1000 and $353 for the 2500ml.

The whole setup looks like this:

Like vulture said, the stills can be used for both storage and distillation, and they have septums from which to draw solvent off.
Not all chemicals are bad. Without chemicals such as hydrogen and oxygen, for example, there would be no way to make water, a vital ingredient in
beer.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Yes, those are the kinds of stills I mentionned I didn't want to have  rather
than have one still per solvent, and roughly 250-500ml disponible at all times, I prefer using the same still batchwise for any solvent, and collect
500-1L in the respectives schlenks/bottles to store (enough to use in a month for solvents in schlenks/under argon) rather
than have one still per solvent, and roughly 250-500ml disponible at all times, I prefer using the same still batchwise for any solvent, and collect
500-1L in the respectives schlenks/bottles to store (enough to use in a month for solvents in schlenks/under argon)
But it's interesting to see each ones' opinion on these. I suppose their implantation is now worldwide 
I have a message from a colleague telling me he located one of the old stills, so I might take a pic or borrow it to show it to the glassblower. I
will post the picture here, so than people can see what I'm talking about.
El, have you installed such a still in your home-lab? Or do you use your regular distn set for solvents?
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Even under argon, dry solvents have a short shelf-life. Storing them should be avoided. Especially THF, ether and solvents like DMF are very
hygroscopic when dry. The ethers will also attack septa over time and make them brittle.
If you must store them, add molecular sieve to the storing flask, and seal the septum with parafilm/teflon tape and grease. Also make sure it's under
slight argon overpressure.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Well, at work we always used to keep these sealed schlensk for up to 1 months, some other MS or CaH2. When stored, we use glass stoppers with minimum
grease, with parafilm, and swap to septums when needed. Obviosuly degassed and purged at each handling. Never had any problems this way, even with
pretty sensible reactions.
But now I guess fresh solvents are even better, even though having "public" stills is somethime frightening when you see how some people redraw from
them... I always felt more confident when i had distilled the solvent myself in a schlenk only me and my labmates handled.. But you don't stop
progress 
EDIT: anyway, for my personal use, I don't need extra pur solvents. The most "extrem" would be for random grignards and LiAlH4 reductions.
[Edited on 6-7-2008 by Klute]
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
domaani
Harmless

Posts: 10
Registered: 15-3-2008
Location: Finland
Member Is Offline
Mood: No Mood
|
|
I spent an hour yesterday playing with glassware, trying to compose a somehow reasonable solvent still with a resevoir as seen on the previous posts.
I have a few ordinary variable reflux heads but the receiver seems to be problematic, almost having to consult a glassblower  It would be a nice feature to have some distilled dry solvent waiting in the
receiver, but it also should be possible to let the solvent flow back to the flask if having been stored for too long. This feature seems to demand a
receiver with a three-way valve. It would be a nice feature to have some distilled dry solvent waiting in the
receiver, but it also should be possible to let the solvent flow back to the flask if having been stored for too long. This feature seems to demand a
receiver with a three-way valve.
|
|
|
NERV
Hazard to Others
  
Posts: 152
Registered: 22-9-2002
Location: USA
Member Is Offline
Mood: Fluorinated
|
|
Considering the cost of waste disposal, and purchasing new solvents I have always been the type to recycle all of my solvents. The system I use is
pretty simple it consists of a fractioning column mounted on whatever size RB flask is necessary for the volume of solvent being recycled on top of
that is a 500ml solvent recovery flask with an Allihn condenser. I simply allow the solvent to reflux over a suitable drying agent in an argon
atmosphere for approximately 24hrs at which point I drain the solvent off into its appropriate container with a constant flow of argon running over it
(depending on solvent volume this can take several times). This setup works great and I have never had a problem with the purity of my solvents using
it (Confirmed with GC/MS).
Here is a picture of the setup that I just put up today to recycle some DCM I had been storing for awhile. I first pre-dried the DCM over magnesium
sulfate then filtered it into the 2 liter flask where it is now refluxing over P2O5. I will collect the purified solvent tomorrow over either fresh
P2O5, or I am considering using 4A molecular sieves.

Vir sapit qui pauca loquitur.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Thanks for the pic! I am definitavily getting more and more jelous of you guys 
can you store your DCM over P2O5 without any discoloring? I guess it will contain some acidic impurities though?
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
NERV
Hazard to Others
  
Posts: 152
Registered: 22-9-2002
Location: USA
Member Is Offline
Mood: Fluorinated
|
|
Yes you can store the DCM over P2O5 without any discoloration of your solvent. The reason there is discoloration in the refluxing flask is due to the
fact that even with pre-drying over MgSO4 there was still an excess of water, and I have a sneaking suspicion that there was a little bit of MeOH in
the DCM as well thankfully the P2O5 took care of that for me  . .
I don’t think that there would be any acidic impurities in the DCM so long as it was completely anhydrous; although the possibility does exist hence
I will be storing the DCM over 4A molecular sieves. I was personally surprised that DCM even had an azeotrope with water; I thought that it was far
too non-polar to absorb any appreciable amount of water.
This entire procedure can be found on page 7 of the attached document. I of course made a few modifications to accommodate my situation.
Attachment: purification of solvents for electroanalysis page 7 DCM.pdf (213kB)
This file has been downloaded 2958 times
Vir sapit qui pauca loquitur.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
I have mounted a small solvent still with my Dean Stark. It works without any problems, i am currently using it to recycle some acetone. Actually I
realized the sytem I was looking for is very similar to this, so I might consider getting a 29/32 dean stark and athermometer adaptor.
I attached a 600mm vigreux on a l three nack RBF, the dean starl on top, mounted with a short alhin condenser protected by a CaCl2 guard (not really
necessay here). The exit from the tap was fitted in a thermometer adaptor, with the adequate joint (the same one I use when placing a gas admission
tube for example), itself connected to a 500mL three nack RBF. The only inconvienment in that the receiver isn't pressure equilized, so it needs some
optimization for distillations under argon (with a schlenk I suppose).
The pre-dried over K2CO3 acetone (600mL) is been refluxed over K2CO3 (~5g) for an hour to get close to equilibrium in the vigreux, then the dean
stark stopcock will be slightly opened to give a low reflux ratio (no special volatil compounds here).
The whole setup:

The Dean Stark:

Reflux:

The pot:

\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
DNA
Hazard to Others
  
Posts: 191
Registered: 11-6-2003
Location: @moon
Member Is Offline
Mood: Experimenting
|
|
@ Klute,
I've got a dean&stark trap but mine looks different then yours mine is completely horizontal and doesn't have this slope in it like yours does.
Anyway what is the point of the reflux ratio, once the solvent is dry why not just collect it?
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Completly horizontal? I don't get the picture. Is it simply shaped as an inverted U? Or just a side arm?
The point of the reflux ratio is that the solvent is passed sevral times over the dessicant, having a better efiicienty. It is roughly the same
principle as the "modern" solvent stills, in that the solvnet that can be collected is always freshly distilled: it the same here, except that it is
always gradually collected.
For recycling solvents, the advantage is more marked: it ables to have a better seperation even while using a vigreux column. For best results, i
would us a long, packed column of course.
Of course, for common grade solvents, a simple distn is often enough, but when drying THF or other solvents for sensitive applications, it is a marked
improvement.
One advanatge from the new stills, is that you don't need to have coutless schlenks in each lab, on still at each floor and anyone can come and
collect with his syringe. But seeing of some guys collect (leaving air come in, letting solvents run down the stilll "oH, it will evaporate before it
reaches the mantle, don't you worry", i prefer doing as I was told to: "Never share syringes!"
Even the guys that need larger amounts of solvents for reactions use the stills earliy in the morning or late at night to fill a few schlenks, and use
after.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
DNA
Hazard to Others
  
Posts: 191
Registered: 11-6-2003
Location: @moon
Member Is Offline
Mood: Experimenting
|
|
Okay it is getting a little bit more clear now, if I understand correctly you have recycled solvent which you would like to dry in first place and
that's why you're using this system.
Second thing is your recycled solvent might still contain another solvent and because of that it is better to gradually collect it?
I mean now you're telling that at your work they have a still on every floor for a different solvent.
Or do you mean you would like to add a mixture of solvents in the still and distill them batch wise and also dry at once.
My dean&stark is like below:

[Edited on 2-9-2008 by DNA]
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Well, for my use is predominantly to recycle solvents contain other solvents too. A reflux ratio increases the seperation between tow constituants (Mc
Cab and Thiele).
A reflux also enables better drying of a commercial grade solvent to anhydrous/degassed.
At work, with have constant-recycling stills, like the pictures posted at the beggining of this thread, to always have a supply of freshly-distilled,
constantly recycled solvent to be withdrawn in a syringe or a schlenk.
I do not do the seperation of recylced solvents and drying at the same time: I only use commercial solvents for drying purposes, to be sure of the
purity. The other recycle dsolvents I keep for extarctions, chromatography, and not specificly sensible reactions, and acetone for cleaning/drying
purposes.
Imagine if I have traces of methanol in my anhydrous DCM and I want to perform an acylation? As I don't do these types of reactions very regularily,
and in smalls clae, i can afford to use new solvents each time, but they need to be distilled to remove any traces of water and oxygen.
Do you use commercial solvents directly for grignards, LiAlH4, etc? Or just straight fractionnation over some dessicant?
EDIT: I suppose your Dean Stark is pretty simialr, as long as the reflux goes back to the still easily. Funny though, I have always seen them inclined
like mine. DOn't you get any drops of water stuck in the horizontal part? Or do they get washed away anyway?
[Edited on 2-9-2008 by Klute]
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
DNA
Hazard to Others
  
Posts: 191
Registered: 11-6-2003
Location: @moon
Member Is Offline
Mood: Experimenting
|
|
I use commercial solvents for LiAlH4 reductions but I do pour in dry 4A molecular sieves.
I also have water droplets in the horizontal part of the dean&stark trap.
So what you are using your still for is to just dump in a mixture of solvents of all kinds and then fractionally distill it and save them.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
No, no, not to that extent 
But I keep all the solvents I use for extarctions and chromatography, once distilled off, in seperate bottle according to the main constituant: one
mainly toluene, one mainly DCM, one mainly AcOET, etc
then, depending on the solvent, I do a couple washes (to remove most esters and alcohols off toluene or DCM for example), acids and alcohols from
ethyl acetate, ex, then distill them int he still, with a little dessicant sometimes (mostly NaOH/KOH to destroy any ketones, K2CO3 fro acetone,
etc). So the solvents are often of technical grade, containing <5% impurities, but sometimes even purer than before (toluene). All these can be
used for chromatography (large consumption), extractions, or reactions that only require regular grade solvents.
What can be annoying are mixture forming azeotropes, or have very clsoe bp's, and that can't be seperated using washes or chemical means (methanol and
acetone). These I keep for cleaning purposes mostly.
It's a bit of work, I've got dozens of used solvents bottles, but I only generate under 5l of organic waste every 2-3 months, compared to every day at
work  and it saves me alot of expense solvent-wise. and it saves me alot of expense solvent-wise.
One day I'm thinking of setting up a larger still, a bit like Stoichiometric_Steve's one, that would turn 12/24H so that I ca ncatch up all my "to
recycle'" work 
[Edited on 2-9-2008 by Klute]
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
tapira1
Hazard to Others
  
Posts: 168
Registered: 9-10-2006
Location: Here!!!
Member Is Offline
Mood: 
|
|
Storing DCM over P2O5
Have read a previous message on storing DCM over P2O5. This cannot be done for much timew, as DCM reacts with P2O5. Usually P2O5 is added to dry the
solvent (by reflux followed by distillation). If you need anhydrous DCM, reflux it 4 h over P2O5 and then distill it (store in Young ampoule or over
MS). If you want to have anhydrous DCM "freshly distilled" it is preferably to prepare it by distillation from CaH2.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Ok, I've finally upgraded to the new fancy still heads like everyone now days 

Hopefull this beauty is going to be up and distilling in a few days! The much larger volume will help me recycle all the used solvents that have been
accumulating, and I will be able to prepare some rigorously dried solvents much more easily.
I'm going to figure out a way of connecting a schlenk or a RBF to the lower exit, to be able to distill the solvent in a receiver batch-wise aswell as
having a constant supply of freshly distilled solvent.
Good point is I will also be able to use the Dean Stark to prepare "technical" solvents from the used ones at the same time. i will need to upgrade
the cooling system though, as even after distilling acetone for a few hours the water heats up to over 30-35°C (roughly 15L in a cooler, with a small
aquarium pump).
And the best thing is that I got for 25E  
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Are you sure about DCM and CaH2? Wouldn't that produce carbenes? I remember someone in our lab trying this and getting some very funky results.
|
|
|
| Pages:
1
2 |