| Pages:
1
2 |
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
Asymmetric Etherification of 1,4-dihydroxybenzene
I've been trying to synthesize 1,4-dimethoxybenzene via 1,4-dihydroxybenzene (hydroquinone). Now before any say that this can easily be achieved using
other methods - of them I am aware.
My concern is moreso that it seems theoretically possible to synthesize 1,4-dimethoxybenzene using much more accessible and safe reagents - namely
methanol and sulfuric acid.
So, I figured that as a protonated phenol is a species that is less prevalent than a protonated alcohol, a simple etherification would work, in which
the CH3OH2+ intermediate is generated, which hydroquinone would then attack, ultimately yielding the phenol methyl ether:
H2SO4 + CH3OH --> CH3OH2+ + HSO4-
CH3OH2+ + HO-Ph-OH --> CH3O(+H)-Ph-OH --> CH3-O-Ph-OH
CH3-O-Ph-OH + CH3OH2+ --> --> CH3-O-Ph-O-CH3
I have run a number of reactions which seem to imply that the reaction works, but I cannot seem to isolate the product:
"Sn2 type alkylation via methyl sulfuric acid and phenoxide"
.09mol HQ (10g)
.18mol CH3HSO4 (~23g - 7.2ml (.18mol) CH3OH + 10.3ml (.18mol) 93% H2SO4)
.2mol KOH (11g)
30ml water, 30ml methanol
HQ was dissolved in methanol and KOH dissolved in water was added. The solution was heated to a light reflux and methyl sulfuric acid slowly dripped
in. The water/methanol/DMB? was steam distilled into a brine solution. Only methanol distilled, and upon acidification a dark red/brown solution was
obtained that did not extract with toluene.
Note: I found a report which said that 1,4-DMB may be produced in a similar manner, but clearly one must follow that procedure
exactly for it to work.
-----
"Sn2 type alkylation via methyl sulfuric acid and phenol" or "Acid catalyzed asymmetric etherification" Run 0
.09mol HQ (10g)
.18mol CH3HSO4 (~23g - 7.2ml (.18mol) CH3OH + 10.3ml (.18mol) 93% H2SO4)
1mol CH3OH (40ml)
HQ was dissolved in 40ml methanol and slowly dripped to a heated solution of methyl sulfuric acid (~110*C), and the mixture distilled slowly. *LOTS*
of dimethyl ether was generated. The distilled fraction was diluted with water to yield ~.5ml of a yellowish oil that smelled like DMB. The reaction
mixture was diluted with water and yielded ~2.5g visually (spilled into oil bath and not isolated  ) of crude, brown/red DMB. ) of crude, brown/red DMB.
Note: This is basically when I decided that a "regular" etherification is actually occurring and not any sort of strange mechanism
via methyl sulfuric acid (although, it might be an intermediate). These are also the conditions in which I may have gotten and isolated (if I didn't
spill into an oilbath) a workable amount of 1,4-dimethoxybenzene.
-----
"Acid catalyzed asymmetric etherification" Run 1
.09mol HQ (10g)
.09mol H2SO4 (5ml 93% H2SO4)
1.54mol CH3OH (62ml)
HQ was dissolved in 40ml of methanol and added slowly to a solution of 5ml sulfuric acid and 22ml of methanol held on an oil
bath whose temperature was 150*C. The rate of addition was kept so that the volume of the solution was stable throughout.
The obtained distillate was diluted in water... nothing happened. Upon cooling the reaction mixture separated into two layers. This was diluted with
water, still forming two layers. In an attempt to extract the DMB immediately, KOH in water was added, but the solution very quickly turned black
(seemingly very rapid polymerization or oxidation) and soon a layer was impossible to obtain. But the yield seemed to be very good (of DMB), and the
solution smelled very strongly like DMB. In short the reaction probably worked but the DMB must be isolated without basification, and then purified
via water/distillation.
"Acid catalyzed asymmetric etherification" Run 2
.18mol HQ (20g)
.18mol H2SO4 (10ml 93% H2SO4)
3.08mol CH3OH (124ml)
HQ was dissolved in 80ml of methanol and added slowly to a solution of 10ml sulfuric acid and 44ml of methanol held on an oil bath whose temperature
was 150*C. The rate of addition was kept so that the volume of the solution was stable throughout.
This was diluted with water, but no layers formed (reaction was not cooled.... hydrolysis?). The solution was basified and extracted - a very small
amount of DMB was obtained.
"Acid catalyzed asymmetric etherification" Run 3
.18mol HQ (20g)
.36mol H2SO4 (21ml 93% H2SO4)
6.60mol CH3OH (264ml) (44ml (1.1mol) + 100ml (2.5mol) + 120ml)
21ml sulfuric acid and 44ml of methanol was held on an oilbath whose temperature was 170*C until evolution of dimethyl ether
was seen (the methanol/water formed was distilled off). HQ was dissolved in 100ml of methanol, and added slowly to the
methanol-sulfuric acid such that DME was evolved (1-2 bubbles/second) and the volume slightly increased through the addition. Once the addition of the
HQ was completed, an additional 120ml of methanol was dripped into the solution, the excess water and methanol distilled off and DME continued to be
evolved. The additions were done at such a rate so as to keep the volume of the solution increasing a slight amount. As soon as addition was complete,
the flask was removed from the oil bath and cooled to room temperature at which point a 150ml of cool water was added to the solution.
The solution smelled strongly of DMB but no layers formed, and extraction with toluene and evaporation of the solvent yielded no appreciable amount of
DMB.
-----
The general idea behind the proportions used was to "selectively" protonate all the methanol (thereby having unprotonated HQ and CH3OH2+) and then
distill off the extra methanol and formed water, which should drive the reaction to completion. It certainly *appears* to work (and makes everything
smell very nice) but again, no isolation of product can occur.
In short, the dark products seem to occur whenever the phenoxide of hydroquinone forms (I assume this is oxidation of the hydroquinone to the
benzoquinone). 1,4-dimethoxybenzene also seems to be formed, based on the smell at least, but then actually isolating any is problematic. There is
also 4-methoxyphenol formed, but it's easy to separate from the 1,4-DMB.
Also, these conditions may form a small amount of dimethyl sulfate in situ although I am unsure of whether or not it does in reality. In any
event, I would appreciate any advice which could help perfect these conditions as they would prove to be very useful for alkylation of simple phenols.
I will be retrying something along the lines of "Run 0", as it seems that having the conditions which generate *LOTS* of DME are more important than
removing water from the reaction (as per Runs 1, 2 and 3).
[Edited on 12-5-2008 by PainKilla]
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Thank you for sharing your experiments!
I was under the impression that MeOH/H2SO4 would mainly form p-MeO-phenol under acidic conditions? The black oil seperating on cooling corresponds
pretty well to what happens with the same conditions but a little benzoquinone added. I know the added benzoquinone surely implies a different
mechanisms (maybe at those high temps some BQ is formed from atmospheric/dissolved oxygen).
Why not try to make the mono-methyl ether with the conventional conditions, and then try to furhter methylate it with methyl sulfuric acid, or one
of it's salt, as a first experiment? You would see if it is even possible to methylate the phenol with only MeOH/H2SO4. If it works an dyou can
isolate a reasonable amount of the dimehtyl ether, you could go back and try a one-pot reaction (excluding the BQ, as it seems to supress formation of
DMB pretty effectively).
Have you tried directly using a salt of MeHSO4? I guess it's lenghty preparation is best avoided, and that's why you try using in-situ MeHSO4...
Good luck and keep us updated!
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Ullmann
Hazard to Self
 
Posts: 51
Registered: 22-12-2004
Member Is Offline
Mood: No Mood
|
|
I agree with klute the methylated compound you get must be p-Meo-phenol through the presence of some benzoquinone that catalyse the transformation as
it is well know from various patents on the process.
You cannot dimethylate the HQ to the DMB this way. It works only with naphthohydroquinone and with phloroglucinol (this one only for one or two
etherification, never three)....
BTW DMB is a dead end unless you want to do a gatterman reaction, a chloromethylation/sommelet or maybe a modified duff but the yields will be poor in
that last reaction unless you halogenate para first (in fact i dont know that is a wild guess it may still give 50%)... And the other two are quite
toxic and no fun... nonetheless it is a bad intermediate IMHO. p-Meo-phenol is much more versatile as you can use CHCl3/NaOH or HCHO/Mg to place the
formyl then methylation of the phenol should be easy with KMeSO4 or Methyl-tosylate or CH3I. Methyl tosylate of course OTC from Boric acid/MeOH reflux
on tosic acid itself readily avaiable from toluene and - i think - that reagent (methyl tosylate) is particularly more suited. You will have better
luck with that route through p-Meo-phenol nonetheless i am convinced.
[Edited on by Ullmann]
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Sorry for the off-topic, but Ullman, have you tried the methyl tosylate synth from trimethylborate/TsOH yourself? How good were your yields? Or do you
assume it works?
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
All of the reactions I have seen that selectively produce p-methoxyphenol use benzoquinone - that reaction is entirely different from this one because
it involves acetals as intermediates. This is just a simple etherification, so I see no reason why the reaction would not go to completion.
In fact, if you look at the production of p-methoxyphenol, you can see that certain conditions (favorable for etherification as earlier described)
actually produce a fairly substantial amount of 1,4-dimethoxybenzene in addition to p-methoxyphenol. Thus I see no reason for this reaction to not
proceed to completion - and it certainly does seem to work... I will retry run 0 in a few minutes and see if I can avoid spilling stuff into an oil
bath. 
The black "oil" is not quite what I mean. I do know what you mean as I've done the 4-methoxyphenol synthesis a few times, but the oil obtained from
this etherification is actually quite clear - it is only upon basification and exposure to air that a solution turns black. This is just unreacted
hydroquinone oxidising, as a pure sample dissolved in base does the exact same thing.
I do have a bunch of very pure 4-methoxyphenol, I just don't feel like using methylating agents for the reasons stated. However this etherification
does appear to work, and using methyl sulfuric acid I think will not matter, because the reaction will still proceed through the same general
pathway/conditions. It's just a matter of optimizing them.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
This can not be a normal etherification since then you would obtain only dimethyl ether (ArOH is nearly not as nucleophilic as MeOH which, being a
solvent, predominates anyway). Alcohols with phenols in highly acidic media rather give C-alkylation products since at the acidic conditions
required these are the products of the less reversible reaction. Though it is possible that in run 0 where you used methyl sulfuric acid (I
assume from MeOH/Na2SO4/H2SO4?) some 1,4-dimethoxybenzene could have formed I find it hard to imagine this could be made into the main product. The
other runs where you used MeOH/H2SO4 should, in my opinion, not produce any detectable 1,4-dimethoxybenzene. The smell means little to nothing. It
would be best if you could follow with TLC instead. Why not trying in basic media to cleanly get the product using methyl sulfuric acid? This way you
could use water as solvent.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
I realize that ArOH is not nucleophilic enough to compete with MeOH, but I am playing around with the conditions to hopefully make it so. By only
using enough methanol to have it all protonated (1mol H2SO4 - ~1.5mol CH3OH), with enough methanol constantly added as the ether and methanol are
removed, all of the hydroquinone should be converted to dimethoxybenzene with a long enough reaction time. As just about all of the reactants can be
recycled, it makes this procedure particularly attractive, even if the yields aren't superb.
In fact, I did redo run 0, and seem to have isolated a small amount of 1,4-dimethoxybenzene (~3-4g) from 10g of hydroquinone (am currently working it
up, will have more details soon). I also generated a very large amount of 4-methoxyphenol (~5g), and there was a small amount of hydroquinone left
unreacted. In short, I think with a long enough addition time of methanol it is entirely possible to forcefully shift the equilibrium towards the
right.
I will try the methylsulfate alkylation if this does not work, but this truly seems promising. I did find a patent that suggested something like this
could work (but I don't have it with me), and it does appear to be...
"Acid catalyzed asymmetric etherification" Run 4
.09mol HQ (10g)
.18mol 93% H2SO4 (10ml)
120ml methanol
Hydroquinone was dissolved in 40ml methanol and 10ml sulfuric acid and slowly heated on an oil bath whose temperature was ~170*C until dimethyl ether
was evolved. 80ml methanol was then slowly dripped in so as to keep the rate of ether formation relatively constant (1-2 bubbles/second). Once the
addition was complete, the reaction mixture was cooled to room temperature, quenched with ice water (resulting in a large amount of tan precipitate),
basified and extracted with toluene.
I'll update as soon as I remove all the toluene.
Note: This reaction seems to work best when it's sulfuric acid and methanol (~1:~1) and a dilute HQ solution in methanol is slowly added to this
heated solution. But I will see for sure soon...
Edit: After evaporation of the solvent I got 1.2g of a dark yellow oil that melted at about 54-56*C. It looks like there is a small
bit of impurities but one should vacuum distill the dimethoxybenzene anyway.
So thus yield was not as good as originally estimated, but still significant considering these conditions appeared to be less than ideal and there is
much room for improvement. I will try next time to start using a small amount of 4-methoxyphenol, for it will then be absolutely clear just how long
the reaction needs to be run (and if it can be tuned to work well at all).
Edit 2: Good news! I just recycled the methanol and upon diluting the last bit I got a bunch of very pure, white crystals of DMB! I
figured that the steam (well, steam/methanol) distillation would be pretty slow and that a significant amount would not carry over but it appears that
it does and it looks VERY pure... I will take a weight in just a moment, but this makes this reaction extremely promising!   
Edit 3: The distilled 1,4-DMB weighed .5g. This isn't significant, but given the high amount of 4-methoxyphenol that also formed, it
appears that a longer reaction time will afford very pure 1,4-dimethoxybenzene using conditions similar to those in runs 0 and 4. The ideal system for
maximum efficiency would be as in the picture:
[Edited on 14-5-2008 by PainKilla]
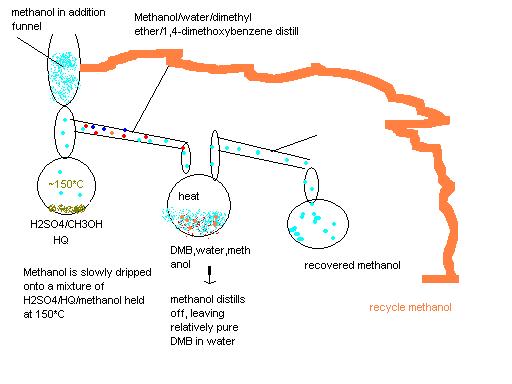
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
Victory! A Methylation Using Methyl Bromide
I saw on another forum that methyl bromide was used to methylate hydroquinone, so I decided to try this out myself. Unlike DMS or MeI, MeBr is cheap
to make (DMS costing time, and MeI costing money). While I had hoped to not use a traditional methylating agent, it turns out methyl bromide is
actually not very toxic!
So anyway on to the good stuff:
110g hydroquinone (1mol)
450ml methanol
150g KOH (2.7 mol)
412g NaBr (4mol)
300ml water
250ml methanol (5mol)
230ml H2SO4 (4mol)
In a 1L RBF flask equipped with a dropping funnel and set up for distillation, methanol and sulfuric acid were slowly mixed together with cooling, and
dropped onto a boiling (saturated) solution of sodium bromide over the course of two hours. The water/methanol/HBr (very little formed) vapors were
condensed into a sealed flask, and the methyl bromide was collected with a plastic tube connected to the vacuum adapter, which led directly into a
solution of hydroquinone in methanol. The methyl bromide was first slowly released to displace the oxygen in the flask, and potassium hydroxide
(flake) then quickly added with *good* stirring while cooling the flask on ice bath. Methyl bromide was then generated (controlled by rate of
MeOH/H2SO4 addition) more rapidly and driven through the solution until the supply was exhausted (2 hours), with vigorous stirring.
The solution first turns brownish (oxidation of hydroquinone) with a yellow tint (phenoxide of 4-methoxyphenol) and eventually a large mass of whitish
crystals form (potassium bromide).
The potassium bromide was filtered (the solution is completely neutral at the end), washed with methanol and dried. 250g was recovered (79% recovery).
About half the methanol was then evaporated (~200ml) and the remains were quenched with 1.5L water and cooled to 5*C. The precipitated clear/white
crystals were then filtered, washed a few times with warm water, vacuum filtered and dried.
Yield: 114g (83% yield)
The obtained DMB melts at 54-56*C.
In short, this methylation is very easy to perform, non-toxic and high yielding. It reportedly works for other substrates also, and I would be
interested to see if it can work on deactivated substrates. In any case, this is definitely the way to methylate simple phenols!
Edit: Just to clarify, the reactions previously mentioned (the H+ catalyzed etherifications) are still seem promising, although they
still need some more optimization. If anyone else wants to pick up where I left off, I would recommend redoing run 4 using more methanol, perhaps in a
setup as shown so as to allow recycling of the materials (as per the picture). However, given the ease of the methylation with methyl bromide, I will
close my lab book in regard to the synthesis of this specific ether using that method.
[Edited on 15-5-2008 by PainKilla]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Excellent job! 
For years I was wondering why nobody reports using this method for methylating phenols while it is the most obvious choice for those who don't have
any dimethyl sulfate or methyl iodide. I often considered trying it myself, but then picking up the bottle of dimethyl sulfate off the shelve is so
much easier, so I never bothered. I'm glad someone else finally did it. You really should write down a full report for the Prepublication section!
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
I'll try and get some pictures if I have time, although I really don't need more dimethoxybenzene. Maybe I can try using this type of methylation on
another sort of phenol(ic aldehyde) as that would certainly show its potential as a methylating agent.
In any event, I'll definitely try again and document this method as soon as time permits.
[Edited on 16-5-2008 by PainKilla]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by PainKilla
...
In short, this methylation is very easy to perform, non-toxic and high yielding.
...
|
While it is an effective and simple proceedure, I must disagree on the non-toxic part, MeBr and MeI are similar in their toxicity:
| Quote: |
Methyl bromide, labeled with a DANGER signal word, is an extremely toxic vapor. In humans, methyl bromide is readily absorbed through the lungs. Most
problems occur as a result of inhalation. About 1,000 human poisoning incidents caused by methyl bromide exposure have been documented, with effects
ranging from skin and eye irritation to death. Most fatalities and injuries occurred when methyl bromide was used as a fumigant.
Inhalation of 1,600 ppm for 10-20 hours, or 7,900 ppm for 1.5 hours is lethal to humans (8). The lowest inhalation level found to cause toxicity in
humans is 35 ppm in air.
Methyl bromide is a dangerous cumulative poison. First symptoms often are due to damage to the nervous system, and may be delayed from 48 hours to as
long as several months after exposure. This delay, combined with methyl bromide's lack of odor, means that the victim may not realize that exposure is
occurring until much time has passed.
Symptoms of poisoning vary widely. Soon after inhalation of large doses, symptoms may include headache, dizziness, nausea, chest and abdominal pain,
and a dry throat. Three to 12 hours after vapor inhalation, symptoms include slurred speech, blurred vision, temporary blindness, mental confusion,
and sweating. More severe symptoms may include lung swelling; congestion; hemorrhaging of the brain, heart, and spleen; severe kidney damage; and
numbness. Death may occur within 1-30 hours, usually from respiratory failure.
Although skin absorption is not an important route for methyl bromide intoxication, the skin is affected by contact with this chemical. Methyl bromide
can cause enormous blisters that are rarely deep enough to destroy the entire skin layer. Small amounts of skin or eye contact brings on shortness of
breath and itching. If absorbed through the skin, nausea and vomiting may result. Clothing that can not "breathe" may delay the evaporation of the
pesticide from the skin. Continued contact with skin can cause death. Ingestion of methyl bromide may cause hand tremors, and convulsions.
The inhalation LC50 for rats is 3,120 ppm/15 minutes (7), 2,700 ppm/30 minutes, and 1,164 ppm/60 minutes. For guinea pigs, it is 300 ppm/9 hours and
it is 2,000 ppm/11 hours in the rabbit (7). The rat LD50 administered in liquid is 20 mg/L (11). The dermal LD50 for rabbits is 15 ppm.
|
| Quote: |
In DNA-binding studies performed in rats and mice, 14C-labeled methyl chloride was given by inhalation, and methylation of DNA bases was examined. The
compound did not lead to specific DNA adducts. In particular, methylation of DNA bases was not observed. In contrast, methyl bromide and methyl
iodide, upon oral and inhalation administration to rats and mice, caused systemic DNA methylation.
http://www.ncbi.nlm.nih.gov/pubmed/8260067 |
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
First of all, a big Bravo to Painkilla! You are the firt person I hear using MeBr in amateur settings, and getting it to work! Indded the preparation
looks simple enough!
I totally agree with Nicodem's suggestion of posting this in prepublication, if you can make a somewhat detailed write-up. It would be evry
interesting to see if it works with phenolic aldehydes, I see no reason why it wouldn't, considering that MeI works perfectly, and TMP also gives very
good yields while been much less reactive. You might need to find a way or keeping the MeBr into solution a bit longer though (dissolving it into a
solvent? Sealed apparatus?) or use a healthy excess, which isn't a problem considering the price of KBr and the 80% recovery you achieved! I think
this is a breakthrough (the whole use of MeBr, not the KBr recovery  ) )
Hum, indeed, I don't think you can use the word "non-toxic"... On the other hand, With a little more precautions, this can be safer than using/making
MeI.
I would use a conc. NaOH wash bottle, or NH4OH, flush the system with argon or nitrogen, add the KOH to the phenol as an alcoholic solution, in the
cold (to avoid quinone/side-reactions) and with good stirring, then start generating MeBr. The fact that it is formed in-situ can mean 0 exposure if
done correctly! Just add NH4OH to the NaBR/H2SO4 solution at the end, purge the system with a slow flow of air (sending all the MeBR vapors in the
washbottle), and you are fit for work up!
And if your compound isn't a aldehdye, such as DMBenzene, you can basify with ammonia to destroy any dissolved alkyl halide, a dilute hydroxide
solution should work too if done in the cold and not left for long.
I'm very impressed by your work. Keep up the good stuff, and can't wait to see how this goes with phenolic aldehdyes!
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | | The fact that it is formed in-situ can mean 0 exposure if done correctly! |
Yes, quite true, a little caution and you can avoid exposure; plus there's no storage concerns beforehand.
Klute's suggestions sound worthwhile, even if you can't do the inert gas flush adding the base in solution should help.
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
Thanks for the comments everyone!
As far as the rate of reaction... it actually goes quite fast (as it is *quite* exothermic) - I think in reality one doesn't really need to generate
MeBr for two hours, rather 30-45 minutes would suffice. In addition, one can also run the reaction in the cold (0*C) and the methyl bromide would stay
in solution , though the reaction would probably be pretty slow then.
But as far as recycling is concerned, maybe the MeBr would be used for some other application? How about driving it into a solution of methanol and
NaCH3SO4? No one can complain about easy DMS if it works. 
Otherwise I don't really know what can be done to improve the reaction, I think it's important to evaluate how effective it is for less activated
substrates as we currently aren't aware at how well it methylates such things...
not_important, when I say that the compound is effectively non-toxic, I mean in comparison to the carcinogenicity of methylating agents... Otherwise
it's just like any chemical.
I don't have a hood but what I did was just run another vacuum adapter tube from the MeOH/HQ flask to the window... It was thus effectively a closed
setup anyway.
http://ntp.niehs.nih.gov/index.cfm?objectid=070917F9-D058-F1...
It's definitely not something you want to use as a fragrance, but it's also not as toxic as methyl iodide or dimethyl sulfate (cancer).
[Edited on 16-5-2008 by PainKilla]
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Fleaker and I were discussing your brilliant experiment, when he suggested using the MeBr to make some MeI with iodides in acetone..
Knwoing that alkyl iodides are more reactive than bromides, wouldn't a catalytic amount of I- be helpfull for the more delicate substartes, such as
phenolic aldehydes? IIRC, this trick is often used in alkylations using alkyl bromides... I also think alkali iodides are more soluble in acetone
than alkali bromides, so this difference could help displacing the equilibrium.
The MeBr could also be used to make methyl tosylate, by passing a slow stream into a suspension of a salt of tosic acid in a adequate solvent,
filtering the bromide salt, and adding the obtained solution to the substrate. This would mean diminsihed toxicity, and the possibility of reflux
without loosing the product through the condenser, though it's another step added. IIRC methyl tosylate and methyl bromide are of similar reactivity,
if not more for the ester, so it could be avantageous.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Methyl bromide is not much less electrophilic than methyl iodide. They are more or less similarly reactive. Only methyl chloride is way less
electrophilic. For every methylation where MeI works, MeBr can surely be used as well. (Coincidentally, MeBr is used as a standard electrophile in the
measurement of relative nucleophilicty of test nucleophiles.)
Just think about the alkylations with EtBr or PrBr which work perfectly well on phenols even in refluxing ethanol (not even DMF is needed!). And the
reactivity of alkyl bromides follows the general SN2 direction: MeBr > EtBr > PrBr >> i-PrBr...
You can not prepare MeOTs from MeBr and a metal tosylate since the bromide ion is much more nucleophilic than the tosylate ion. Such a reaction would
require the use of silver tosylate to push it in the direction of MeOTs, but other metal tosylates can't work.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
I'm surprised of this, I could have sworn I had seen a preparation of tosylate from alkyl halides... I beleive you, but I will need to go through some
articles to see what made me think this works..
It's true alkyl bromides are often used when it comes to 2 carbon chain and more, I'm guessing the difference of prices comapred to the small
difference of reactivity isn't worth the change. I guess with MeBr the iodides could help keeping some alkyl halide in the time it reacts.
EDIT:
Hum, I think I inversed the reaction in my head... The article I had in mind was the one I'm attaching, but indeed it deals with the preparation of
alkyl iodide from alkyl tosylates and NaI.. The other way around...
On the other hand, alkali sulfinates can dispalce halides, maybe that got me confused to, by analogy. (Note 2 from this OrgSyn prep ). Too bad for the idea then...
Article attached:
"The action of sodium iodide on some esters of p-toluenesulfonic acid"
R.S. TIPSON, M.A. CLAPP, L.H. CRETCHER
[Edited on 18-5-2008 by Klute]
Attachment: jo01165a017.pdf (363kB)
This file has been downloaded 1505 times
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Klute
I'm surprised of this, I could have sworn I had seen a preparation of tosylate from alkyl halides... I beleive you, but I will need to go through some
articles to see what made me think this works.. |
I think you found the paper here: http://sciencemadness.org/talk/viewthread.php?tid=10275&...
But silver tosylate is used which makes the reaction toward the ROTs irreversible due to the insolubility and the formation of covalent bond in AgBr.
Methyl bromide is never used for preparative methylations in organic labs simply because it is a gas at room temperature while methyl iodide is a
liquid. So you can be sure everybody would prefer MeI due to practical reasons and not that much because MeBr is somewhat less reactive. After all, in
many solvents dimethyl sulfate is also somewhat more electrophilic than methyl iodide and still both are often used.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
a bit off topic, but i think it fits here since the use of MeBr is discussed:
during the ether cleavage of methyl phenyl ethers using AlCl3/Pyridine, MeCl is formed. is there any efficient way of making this react to something
harmless?
EDIT: I'm stupid. what was i thinking...the MeCl reacts with Pyridine to N-Methylpyridinium chloride.
[Edited on 19-5-2008 by stoichiometric_steve]
|
|
|
ShadowWarrior4444
Hazard to Others
  
Posts: 226
Registered: 25-4-2008
Member Is Offline
Mood: Sunlight on a pure white wall.
|
|
| Quote: | Originally posted by stoichiometric_steve
a bit off topic, but i think it fits here since the use of MeBr is discussed:
during the ether cleavage of methyl phenyl ethers using AlCl3/Pyridine, MeCl is formed. is there any efficient way of making this react to something
harmless? |
Inhalation is an efficient way to accomplish that.
On a serious note, you can likely react it with chlorine gas at 400-500C to produce progressively more chlorinated versions. A mixture of methyl
chloride, DCM, chloroform, and CCl4 should result, which would then be separated by distillation. You may also want to run it though a gas liquefier,
bottle it, and use it as a refrigerant.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
MeCl is much less toxic than MeBr.
Anyway, a good way to render alkyl halides and alkylating agents in general harmless is to add them to ammonia.
Ammonia and amines are rapidly and easily alkylated, consuming the alkylating agent.
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
Another interesting modification may be to perform the reaction at a low temperature (<3*C) so as to keep all of the methyl bromide in solution.
While the reaction rate will be slower, the concentration of MeBr should be quite high, thereby promoting the reaction... I would think that MeBr is
also fully miscible with methanol (or basically any Sn2 solvent) so this would also make removal of the gas non-problematic (as it would just be a
liquid in solution).
The only potential problem with this is that the reaction (in the case of hydroquinone) is quite exothermic, enough to keep the methanol at 50-60*C
(estimated). Granted, this could be fixed by using very efficient cooling but I think the easiest thing to do would be to just to use a longer
reaction time, letting MeBr enter the reaction flask at a slower rate.
I think next time I will also add hydroquinone to KOH in methanol (instead of the other way around), as this should help to prevent oxidation
(although this problem is pretty much unique to hydroquinone).
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
| Quote: | | I think next time I will also add hydroquinone to KOH in methanol (instead of the other way around), as this should help to prevent oxidation
(although this problem is pretty much unique to hydroquinone). |
Hum, I don't totally agree. I've witnessed that nearly all phenolates are readibly oxidized by atmospheric oxygene even at RT. This can be seen by the
difference in colour of the medium if the formation is done under argon or not, all other conditions been equal.
With salicylaldehdye, the phenolate is orange/yellow under argon, but quickly turns dark brown/black when done under atmospheric conditions. This
also happens with phenol to a lesser extent, aswell as other non-aldehydic phenols.
That's why i always prefer adding the base to the phenol, or the other way around, at cold temperatures (0-5°C), under argon, and over 10min,
leaving it to stir for another 10-15min.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
I think salicylaldehyde is a bad example to use though, I mean orthohydroxy aldehydes tend to polymerize fairly easily from what I've seen in the
literature, and I haven't seen so rapid a change with any plain phenols. Even hydroquinone oxidises pretty slowly.
Anyway, the idea would be to add the hydroquinone to a flask with air displaced by MeBr. You'd lose a bit of MeBr initially but then since you don't
lose any later on, you can use a much smaller equivalent of all reagents and on top of that, be able to recover most of the bromide put into the
reaction.
Gas management becomes a non-issue then too!
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
In my experience, aqueous solution of sodium phenolate is stable for weeks. It does darken a bit after a couple of days, but the oxidation products
are not detectable on HPLC, so they must be only in ppm concentrations.
With hydroquinones (or catechols) the most problematic is not the oxygen in the air above the solution, but the oxygen dissolved in the solvent used.
Oxygen need some time to dissolve, so it is mostly the already dissolved oxygen that causes the darkening seen initially. Thus it is best to degas the
solvents prior use. This can be easily done by letting it stay for a minute under the aspirator vacuum or to heat the solvent to boil. Another often
used trick is to add a pinch of sodium dithionite in the reaction mixture. This small amount does not interfere with the alkylation but it does reduce
all dissolved oxygen and all of the quinones formed by oxidation.
Ethanol or isopropanol should absorb MeBr gas more rapidly than methanol even when warm. And both are also better solvents for SN2 reactions since
they solvate the phenoxide anions considerably less so than methanol (unsolvated anions are much, much better nucleophiles, since solvation is the
main factor in increasing the activation energy of the reaction).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
| Pages:
1
2 |
|