jhd5000
Harmless

Posts: 6
Registered: 11-4-2008
Member Is Offline
Mood: No Mood
|
|
Organoboron enolization reagent for a tertiary amide
I am working on an organic chemistry synthesis and have run into a bit of a snag. I am trying to synthesize an imidazolidinone chiral auxiliary
(similar to the Evans), followed by alkylation with isobutryl chloride, followed by using (n-Bu)2BOTf as a boron enolization reagent and alkylation
with benzaldehyde in one pot and octanal in the other. After cleavage, the auxiliary will be cleaved and the optical activity of the products
determined in order to determine stereoselectivity. I can not get access to (n-Bu)2BOTf, however, and I was wondering if something like LDA might be
an adequate substitute. Any suggestions? I attached a copy of my outline and key reaction for more clarity.
Attachment: Team_Outline[1].doc (359kB)
This file has been downloaded 682 times
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Dibutylboron trifluoromethanesulfonate is commercially available a Acr*s.
You can use LDA in THF (ketyl dryied) in dry ice/acetone to form the enolate, but mind that in such a case you will have a base formed lithium enolate
instead of the acid formed like with Bu2BOTf which means you might get a much lower or at least very different chiral induction. Only an experiment
can tell you if LDA is really suitable to your requirements. Also, making enolates of such a particular system via LDA is not very straightforward and
requires some know-how. You will have to do it several times to get optimal conditions (there are many papers on N-acyl-imidazolone enolate chemistry
and using them as chiral auxiliaries so check them for details). I think one of the side reactions is the cleavage of the imide into
N,N-diisopropylamides and so on. Overall, personally I would stick to the method using BuNOTf assuming it is published and verified especially in
regard to the chiral induction issue.
PS: It might be better if you just attach the scheme as a GIF picture so that it gets displayed on page. Some users might worry to downloading World
files. (by the way, the enolate-boron complex in the center lacks a double bond)
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
ziqquratu
Hazard to Others
  
Posts: 385
Registered: 15-11-2002
Member Is Offline
Mood: No Mood
|
|
Much of the stereoselectivity given by Evans' reagents depends upon complexation of the Lewis acid to the auxiliary, the attached acyl group and the
aldehyde you're adding. This controls a variety of things, such as which enolate is generated (E or Z), and how the transition state between the
enolate and aldehyde forms. In fact, by choosing the right conditions (choice of Lewis acid, and ratio of Lewis acid to base) you can get very high
control over the generation of the syn or anti aldols. LDA, whilst suitable at face value, doesn't give this added level of control, so your
stereoselectivity may suffer.
In our lab, we use Oppolzer's sultam as our standard auxiliary, and we use diethyl boron triflate (made in situ from triethylborane and triflic acid.
Basically, add mix equimolar amounts of Et3B and TfOH[add the TfOH dropwise with good stirring]. Stir for 30 min - you should get a homogeneous
solution. If not, heat it in warm water - 40-50 *C - for anouther 30 min. if it still hasn't become homogeneous, start again) along with Hunig's base.
This generally works quite well, and I know Et2B works with Evans' reagents too. It's convenient because most labs would have access to both Et3B and
TfOH, but perhaps not Bu2BOTf. I can dig up some references if you're particularly interested, but they're not hard to find either.
I know TiCl4 is a popular reagent for these reactions - again, this will probably impact the stereochemistry generated - in fact, I think TiCl4
typically gives opposite stereoselectivity (syn/anti) when compared to the same reagents using boron enolates. Whilst it might not seem important to
you (since you only generate one new chiral centre), it might still be important to the stereochemistry of that centre. In any case, something which
can complex your acyl oxazolidinone is probably going to give the best results.
|
|
|
jhd5000
Harmless

Posts: 6
Registered: 11-4-2008
Member Is Offline
Mood: No Mood
|
|
Thanks for the input Nicodem and Ziqqurate. I have found some literatue on making diethyl boron triflate in situ so I think I will opt for that. I
know we have Et3B and TfOH so that should work. Thanks again.
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
| Quote: | Originally posted by Nicodem
You can use LDA in THF (ketyl dryied) in dry ice/acetone to form the enolate, but mind that in such a case you will have a base formed lithium enolate
instead of the acid formed like with Bu2BOTf which means you might get a much lower or at least very different chiral induction.
|
How is the Bu2BOTf enolate acid formed? He uses triethylamine in the scheme*, this enolizes the "imide". As I understand, both enolates would be base
fomed, the only difference would lie in the enolate trap, with LDA it would be lithium and in the case of dialkylboron triflate it would be the
dibuthylboron who will bridge both 1,3-oxygens. So in a sence, LDA would function as both base and trap, while triethylamine requires a trap to be
added (i.e R2BOSO2CF3).
*EDIT: There's an error in the pericyclic transition state, a double bond is missing to form the enol...
[Edited on 19-4-2008 by Sandmeyer]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I don't think Et3N is basic enough to appreciably promote the enolization of the imide. I think it is only added to push the boron complex formation
equilibrium to completion by neutralizing the formed TfOH (which would otherwise interfere with the aldol as well).
I think the mechanism of formation of this boron complex is identical as when using BF<sub>3</sub>, BCl<sub>3</sub> or other
boron acids to form the stable complexes with 1,3-dicarbonyl compounds (thus starting with chelating the carbonyl rather than alpha-deprotonation).
So, in my opinion, with Bu<sub>2</sub>BOTf you don't first get the imide deprotonatated by Et<sub>3</sub>N followed by enolate
being trapped with the Bu<sub>2</sub>B<sup>+</sup>. Rather the opposite, that the Bu<sub>2</sub>BOTf chelates to
the 1,3-dicarbonyls followed by deprotonation by Et<sub>3</sub>N and consequent TfO<sup>-</sup> leaving.
However, to demonstrate the puzzling nature of these concepts, one could actually argue that LDA also causes acid promoted enolization since
Li<sup>+</sup> is a strong acid in less polar aprotic solvents and thus the enolization can start either by lithium cation chelating at
the carbonyl followed by proton elimination or by diisopropylamide anion deprotonating the imide. As you see, what we are taught in school are all
simplifications, while the real truth is too ambivalent to catch all (especially considering that all ionic species, if truly present at all, are
solvated). It is however a funny mental exercise to imagine LDA as an acid!
Nevertheless, I did express badly above. Whether the enolization is acid or base promoted is not important, but how the enol is "trapped" does indeed
have an influence on the diastereoselectivity of the reaction (and thus also on the resulting enantiomeric excess after the auxiliary removal). Even
if you ignore the reactivity differences resulting from the ability of the acid part (Li, BBu2 and so on) to dissociate, you are still left with the
huge steric and electronic differences between them (like the enormous difference between Li and BBu<sub>2</sub> . Like Ziqquaratu said, they make a big difference in practice. I never worked with
these chiral auxiliaries even though they are quite popular. As a consequence, I never read much about them so I'm guessing mostly. I try to stay as
far away from asymmetric synthesis as far as my boss allows me to. . Like Ziqquaratu said, they make a big difference in practice. I never worked with
these chiral auxiliaries even though they are quite popular. As a consequence, I never read much about them so I'm guessing mostly. I try to stay as
far away from asymmetric synthesis as far as my boss allows me to.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Ok, yes, I was bringing up unimportnant point, wheter Et3N works as proton sponge (which is likely) or to enolize is not importnant. These boron
compounds are lewis acids and can complex with carbonyl groups, I understood that was what you ment after I made the post  . .
|
|
|
jhd5000
Harmless

Posts: 6
Registered: 11-4-2008
Member Is Offline
Mood: No Mood
|
|
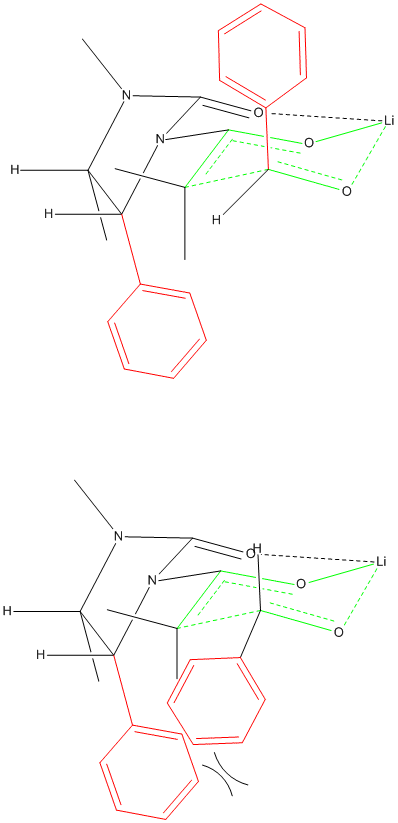
Transition State Chelation
I am trying to represent chelation in the 6-member ring-like transition state of an aldol reaction using a chiral auxiliary. Does this drawing make
any sense?
[Edited on 27-4-2008 by jhd5000]
[Edited on 27-4-2008 by jhd5000]
|
|
|
jhd5000
Harmless

Posts: 6
Registered: 11-4-2008
Member Is Offline
Mood: No Mood
|
|
Those extra Hydrogens shouldn't be there obviously.
|
|
|
jhd5000
Harmless

Posts: 6
Registered: 11-4-2008
Member Is Offline
Mood: No Mood
|
|
I posted the other conformation as well if that helps
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
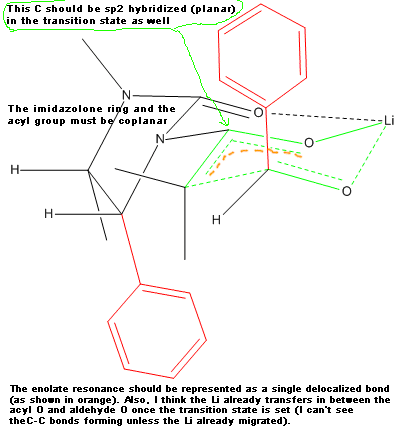
Your transition state has five centered bonds on two adjacent carbon atoms and two 3 centered bonds on O atoms all in the same six membered ring.
This seems like an unusually high energy situation. Is there evidence for it?
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I'm no expert on chiral auxiliaries so do not take my reply as authoritative. I only pointed out things that in my opinion could be wrong, but I do
not claim they actually are. Besides, this forum is for amateur chemistry. If you need a reliable answer for your work related issues, please rather
consult your boss or your peers.
PS: As you see I merged your latest question with your previous one. Please do not open a new thread when you want to discuss the same topic for which
you (or someone else) already opened a thread. There is no need to make searching the forum more difficult than already is.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
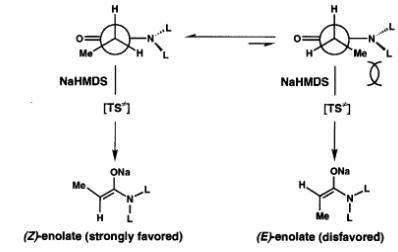
There are examples in the literature where sodium bis(trimethylsilyl)amide (NaHMDS) can deprotonate the imide and trap the enolate by bridging the
1,3-oxygens with sodium (just like you want to do with LDA). For one such example read Evans paper on total synthesis of Cytovaricin (see Evans et al
JACS 1990, 112, 7001). They go on to alkylate the chelated enolate with allyl iodide to obtain the product in 99% diastereomeric purity, however this
is all alkylation and not aldol. To me it seems that the NaHMDS method is alkylation-specific (I suppose LDA might at best be only
alkylation-specific) while n-Bu2BOTf/Et3N combination aldol-specific. I haven't read the above Evens paper, but there is a lot of asymetric aldol and
alkylation chemistry in it. Btw, you can represent conformational control elements much better like using the style in the below picture (this is for
NaHMDS induced enolization).
[Edited on 2-5-2008 by Sandmeyer]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
That scheme seems to indicate the chiral induction happens already at the deprotonation step with sodium hexamethyldisilazane. Is that the case also
when using LDA and Bu2BOTf?
I'm not going to read all the papers on Evans auxiliary, just being curious about it. I also wonder if the aldol with benzaldehyde in jhd5000's
example would then occur with such a high diastereoselectivity given that the deprotonation and electrophilic attack occur on a non-prochiral
position. Without the formation of chirality at the alpha-amide position the chiral induction on the benzaldehyde's prochiral carbonyl would have to
occur over an additional bond length. Ziqquratu, you seem to be familiar with this chemistry. What do you think about it?
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
As I said, it seems that LDA/NaHMDS are used for alkylation while nBu2BOTf is used for addition (aldol). I just found an example (http://www.arkat-usa.org/get-file/19467/) where LDA is used for aldol - but it gives poor diastereomeric selectivity. The explanation why nBu2BOTf
displays selectivity and LDA dosen't is attempted on pages 3-5 in the above pdf file. There is detailed study on the same topic in this paper but I
can't acses it over weekend: http://dx.doi.org/10.1021/ja00401a031
jhd5000 you may also want to request following http://www3.interscience.wiley.com/cgi-bin/summary/114129003... if you want to know more about what you're doing.
[Edited on 3-5-2008 by Sandmeyer]
|
|
|