Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Melatonin: 5-MeO-N-Acetyltryptamine
This ubiquitous hormone was a bit of a health food fad a while back I think it is OTC. It used to be recommended for jet lag and motion sickness, and
way overhyped as an anti-aging panacea.
Anyone interested in the chemistry of melatonin? If so I will discourse a bit.
[Edited on 11-3-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
A user of the german forum has attempted the synthesis of this compound and documented the steps that worked.
He started from 5-methoxyindole and oxalyl chloride.
The problem was at the last step, after the LAH reduction he was not able to isolate any product.
Of special interest would be the preparation of the precursor 5-methoxyindole. Mighty expensive from chemical suppliers.
Do you know something about this?
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I looked at this sort of thing a long, long time ago and have paid scant attention to it since.
The problem in Germany is that I believe melatonine itself is not OTC and is in fact banned. Same with some other places and NZ.
But curiously not USA where it has been OTC since 1993.
Anyway I am a little bored and I thought I might review what is involved in the prep of this.
Initially I will look at these entry points:
Tryptophans and substituted tryptophans - seems to me readily convertible to tryptamines by decarboxylation.
Indole-2-carboxylic acids from o-nitrotoluenes. If this acid can be readily decarboxylated then this sequence could start by nitration of m-cresol to
3-methyl-4-nitrophenol. Methylation at any appropriate stage is trivial. This, eventually, gives the 5-methoxyindole precursor.
Also I have a lot of reading to do to see how melatonin is built commercially.
The 1960 JOC first synthesis of melatonin by two routes, is attached. Both start with 5-methoxyindole, which we have discussed below.
[Edited on 11-3-2008 by Sauron]
Attachment: melatonin.pdf (386kB)
This file has been downloaded 981 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
I am actually quite interested in this compound. I am researching redox cycling antioxidants, so a radical scavenger which is terminal (does not
cycle) is of great interest to me. The trick, I would think, would be the preparation of the stuff for less than $75 US (Aldrich) per gram (a few g is
all I would need).
This thing looks like it could be made to cyclize to yield something resembling a yohimbine-like tricyclic alkaloid?
Cheers,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I dunno about the yohimbine structure as I honestly have forgotten what it looks like.
As you probably surmised I am not particularly interested in the psychotomimetic relatives of this stuff, and while it is true that this thread, if
succesful, might give some aid and comfort to those who are so inclined, I don't care.
I did, within the last year, look at tryptophan preps, and it looked like 5-hydroxy or methoxytryptophans would not be a problem. But my interest in
Trp is purely related to peptide work, so at the time I did not follow the logic out to melatonin.
For 5-methoxyindole the likely starting material is 3-methyl-4-aminophenol. Formylation of the amino group sets us up for a classical indole prep that
is detailed in Org.Syn. (see attached). Some thought needs to be paid to the timing of the methylation of the phenolic function.
The conversion of 5-methoxyindole to 5-methoxytryptamine and on to melatonin is described by two routes in JOC 25 857-858 (1960) which is likely the
basis for the writeup garage-chemist is talking about. I have requested this and will post it here when it is available.
[Edited on 11-3-2008 by Sauron]
Attachment: CV3P0479.pdf (145kB)
This file has been downloaded 1035 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ephoton
Hazard to Others
  
Posts: 463
Registered: 21-7-2005
Member Is Offline
Mood: trying to figure out why I need a dark room retreat when I live in a forest of wattle.
|
|
yummy so close but oh so far oh well indoles can be so fun never thought that kind of chem was your thing. well i shall stand back watch and rethink
my ideals. personally i prefer my tryptamine to
have methyl groups on the nitrogen though and possibly the substitution on the 4 rather than the 5.
[Edited on 11-3-2008 by Ephoton]
e3500 console login: root
bash-2.05#
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Although the Org.Syn.prep posted above employs overpriced potassium metal to prepare t-BuOK, constituting a hazard as well as expense, the authors
mention in the notes that NaOMe can be used and provide reference 14 (JACS 1948) to back that up.
The alternative to 5-methoxy-o-toluidine is 5-methoxy-o-nitrotoluene. From methylation of the corresponding phenol. Aka 4-nitro-m-cresol. This is
commercially available, under $60/100 g. Quite obviously, 4-nitro-3-methylanisole can be reduced easily to the methoxy-o-toluidine. The advantage here
is avoidance of ambiguity in the methylation; we want to methylate the phenol group not the amino group.
With a good quantity of both the methoxytoluidine and the methoxynitrotoluene in hand, both procedures for making the indoles are open and can be
compared. It turns out that the procedure resulting in the indole-2-carboxylic acid (from ethyl oxalate) is useful because the carboxylic acid
function decarboxylates at c.230 degrees.
The Org.Syn. prep of ethyl indole-2-carboxylate is attached.
[Edited on 11-3-2008 by Sauron]
Attachment: CV5P0567.pdf (158kB)
This file has been downloaded 1411 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Quite Alternative Route
US Patent 5,122,535 details the preparation of melatonin from p-anisidine (4-methoxyaniline) as follows:
1,3-dibromopropane is reacted with potassium phthalimide to obtain 3-bromophthalimide. This is treated with sodium diethylacetoacetate.
The resulting acetoacetate reagent is reacted with diazotized p-anisidine at 0 C.
The product is worked up, purified and N-acetylated with Ac2) to melatonin.
The patent is attached.
This has the virtue of going to the tryptamine rather than to the indole. The steps are all well trodden - phthalimido derivative, sodio-acetoacetate,
diazotization, so anyone who is comfortable with those procedures should be confident attempting this overall scheme.
[Edited on 11-3-2008 by Sauron]
Attachment: US5122535.pdf (260kB)
This file has been downloaded 744 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Forgive the repeat post without an intervening reply. Here is the reaction scheme from the 3-bromopropylphthalimide (Gabriel reagent) to the
5-methoxytryptamine with phthalimide still attached and prior to hydrolysis and decarboxylation of the 2-carboethoxy side chain.
The patent details workup, stepwise with 2M NaOH, then 20% H2SO4, to afford impure 5-methoxytryptamine (aka mexamine) which is then purified with
hexamethyldisilane and finally N-acetylated with acetic anhydride to melatonin.
Phthalimide is a potent teratogen, and p-anisidine, a cancer suspect agent. Whether or not curent pharmaceutical practice would find these reagents
acceptable, deponent knoweth not. As always anyone attempting this sequence for preparing recreational derivatives, be warned that rigorous
purification is mandatory, or you may have reason to regret.
[Edited on 12-3-2008 by Sauron]

Sic gorgeamus a los subjectatus nunc.
|
|
|
hempshaman
Harmless

Posts: 4
Registered: 10-3-2008
Member Is Offline
Mood: No Mood
|
|
I remember seeing something where 5-MeO-N-Acetyltryptamine was converted into 5-MeO-N,N Dimethyltryptamine but can't remember where the source was.
Anyone else seen this? The acetyl group needs to be replaced with 2 methyl groups? Is this correct?
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The thread is about melatonin, a nutraceutical. It is inappropriate to derail it to discuss 5-MeO-DMT, Anyway, once again you are asking for
spoonfeeding, when it is obvious to anyone here who is qualified to DO it, how to apply and remove an acetyl group, and how to carry out
N-methylation.
You opened a thread yesterday about 5-MeO-DMT and it was promptly closed by the moderator. My advise is, take the hint.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
The indoles synthesis using that coupling of diazonium salts with acetoacetate esters is called the Japp-Klingemann reaction (just in case this makes searching easier).
There are numerous routes to 5-methoxyindole, mostly researched by Chinese since it is of industrial importance for the pharmaceutical industry. There
are also some relatively simple routes (including the ones starting from m-cresol, nitration, O-methylation and so on), but I
currently don’t have the time to go trough hundreds references.
5-Methoxytryptophol can also be prepared from 5-methoxyphenylhydrazine hydrochloride and 3,4-dihydrofuran, but the yields are unreliable: http://www.erowid.org/archive/rhodium/chemistry/tryptophol.h...
5-Methoxytryptophol can then be mesylated or tosylated, substituted with azide, reduced to amine and N-acetylated. Not a particularly
comfortable route, but if you happen to have the material it is not so bad either.
| Quote: | Originally posted by hempshaman
I remember seeing something where 5-MeO-N-Acetyltryptamine was converted into 5-MeO-N,N Dimethyltryptamine but can't remember where the source was.
Anyone else seen this? The acetyl group needs to be replaced with 2 methyl groups? Is this correct? |
The N-acetyl group in melatonin can not be replaced by methyl groups, it needs to be removed by hydrolysis first. This is not particularly
simple since melatonin gets oxidized very easily. Here you have one example where the hydrolysis is done in under reductive media:
| Quote: | Melatonin (2.2 g, 0.019 mole) dissolved in about 50 ml 1:1 aqueous ethanol was treated with 50 ml 1:1 of aqueous ethanolic KOH in excess (8–12
molar) and catalytic amounts (200 mg) of Na2S2O4. The mixture was heated to reflux under N2 for 16 hr. The reaction mixture was cooled and extracted
with CHCl3. The organic extract was, washed (H2O), dried (Na2SO4) and evaporated to dryness on a rotary evaporator, to give a colorless syrup of
5-methoxytryptamine; this was dissolved in ether and EtOH and treated with HCl in EtOH/ether to give the hydrochloride, 1.7 g of 1 (70%). Mp
243–245°C (dec). Recrystallization with EtOH/ether gave mp 246–248°C (dec).
From: Neurochemical Research, 26 (2001) 1171-1176 |
Now, stick to the topic! The thread is discussing the synthesis of melatonin, not its destruction.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I posted the Organic Reactions chapter on the Japp-Klingmann reaction some time back as it is part of OR v.10 I believe. It's in References, New Book
thread (Organic).
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
I am not the least bit interested in potential psychomimmetic properties of yohimbine (I was referring to the main ring structure, not the compound
itself).
No need for boner pills  . .
My specific need involves a *non-phenolic* antioxidant which does not engage in redox cycling *and* can be followed with a relatively sensitive
technique such as spectrophotometry, or, better, fluorescence.
I'll likely give this a shot if I can find the time (since I need some and have no money, but ample precursors, it may be a forgone conclusion...).
Oops, here's the yohimbine structure for reference.
Cheers,
O3
[Edited on 11-3-2008 by Ozone]
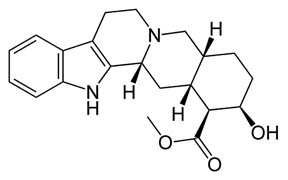
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
A friend, Sauron, contributes:
The Org.Syn.preparations of 5-methoxy indole, precursor of Melatonin, depend on the availability of the appropriate substituted o-nitrotoluene, or
o-toluidine from reducing the same.
Direct nitration of m-cresol is unsatisfactory as the 6-nitro isomer predominates. The following procedure was published in JACS 66, 2019-2020 (1944)
and gives a much better yield, even after methylation to the required 3-methyl-4-nitroanisole:
A stirred solution of 54 g m-cresol in 150 ml AcOH and 20 ml conc sulfuric acid is treated at 8-10 C with 35 g sodium nitrite in a little water. After
a few minutes the mixture is poured into ice-water, and the crystalline nitroso compound that seperates is filtered off. This is added in portions to
a stirred mixture of 50 ml conc nitric acid (d.1.42) and 150 ml water at 40-50 C. Stirring is continued until no more nitric oxide evolves.
The crude nitrocresol is isolated by pouring into water and filtering, is suspended in 200 ml water at 40-45 C . 60 ml dimethyl sulfate is added in
portions alternating with a solution of 35 g NaOH in 90 ml water. The crude product is washed with water, dried and distilled, giving 50-56 g pure
3-methyl-4-nitroanisole, m.p. 55 C.
[Edited on 17-3-2008 by chemrox]
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ozone,
Melatonin is dirt cheap on e-bay. About $10 for 300 3mg tablets (900mg). Distributers seem to be dumping it. Currently, Melatonin is easy to make,
legal in the US, and not hard to come by. As is sometimes the case, Aldrich seems to have it wrong.
Must be really cheap as bulk powder, if you can get away from Aldrich, and
find a better source of supply.
|
|
|