| Pages:
1
..
6
7
8
9
10
..
13 |
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
| Quote: | Originally posted by tentacles
A tiny bit on topic (considering the current discussion of those damned tin compounds) -
Ordered me one of these:
3715 Tin tetrachloride pentahydrate, Purity: min. 98.0%, Grade: Pure, crystalline lumps, Size: 500g $19.00
and
3598 Lead oxide, yellow, Purity: (complexometric) min. 98%, Grade: Reagent, Size: 1kg $28.89
Should be enough SnCl4 to last several lifetimes. Any Canadians that want a dash or two, let me know as I will make my run down south to pick this up
in a few weeks perhaps. I've seen what Canadian chemical suppliers want for (anything), and it's frightening. |
Yeah that should last awhile . Not a bad price . I have some ordered reagents also , but didn't get those prices .
Sorry for the digressions concerning the history of tin chemistry , but it seemed that alternatives to buying
commercial reagents was in need of a look back at ways
of working from the metal or other precursors , to prepare
reagents of whatever sort desired .
S.C. Wack has provided a very good century old working
instructions for preparations for SnCl2 and SnCl4 by several
proven methods . This was posted over in dann2's SnCl4
thread , here:
http://www.sciencemadness.org/talk/viewthread.php?goto=lastp...
and I am attaching the file here also .
My bismuth arrived the other day , yay ....but once again
I am occupied with other business that is keeping me from
experiments . I did find a spray pyrolysis article concerning
the application of defect free films of Bi2O3 , derived from
Bi(NO3)3-5H2O 0.05 molar in glacial acetic acid sprayed onto the substrate at 300C .
Anyway I still think the stannic nitrate and bismuth nitrate
with stannic nitrate mixtures are worth trying as an overcoating for the spinel , and also in combination with the MnO2 . Also I am intrigued by the
possibility of a nitrate derived Pytlewski polymer of tinIV and tinII , and also
tin and bismuth .
[Edited on 21-2-2008 by Rosco Bodine]
Attachment: SnCl4 and SnCl2 Preparations.pdf (242kB)
This file has been downloaded 1335 times
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Well, I have given up on oxidative soak for the moment.
I oxidised some SnCl2 solution with H2O2 (dropwise) and to this clear solution I have added some Bi(NO3)3 solution (with nitric acid), the solution is
still clear and I am using this as a pyrolytic coating directly on to etched Ti. The coating is being baked at about 470 oC. using the hot air gun
with another vent taped over. It is producing a satin grey white coating. It's sort of like the Bi equivalent of Dann2's ATO precursor coating,
perhaps we could call it BTO ... 
It is not really an attempt at a serious anode, I just wanted to see how the solutions behaved and how the pyrolysis went. I'll try it in a
perchlorate cell, though, as Dann2 reported that a plain ATO anode lasted a while.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Just don't throw out the baby with the bath water ,
the spinel is still good , it's the stannous salt that is the problem there preferentially reducing the cobalt oxide
in the same way as are oxide coated anodes vulnerable to
open circuit scenarios . Working with a stannic salt or perhaps adding some anodic potential for protection ,
or even anodic electrodeposition of the SnO2 , as well
as dip and bake schemes could all work okay without trashing the spinel .
A translation / modern interpretation of that Lowenthal article has been posted by woelen in the needed references and translations thread . The
information
aligns very nicely with what I was thinking was significant .
Evidently the reactions which I was rewriting using the more modern formulas to describe what Lowenthal was
probably seeing in 1852 are likely valid . Anyway , my
earlier speculations were probably paydirt , as opposed
to being off on a tangent concerning the significance of that old journal article . Lowenthals experiments indeed do seem to reveal a shortcut to
alpha stannic acid from
tin metal is possible .
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
Rosco: have you read any articles/patents on electrodeposition of SnO2? A quick google brought up a couple hits..
This one is a long shot, the description indicates it's not what we want - porous coatings deposited in a nitric acid solution.
http://linkinghub.elsevier.com/retrieve/pii/S002202480400982...
Here's an article on electrodepositing SnO2-Sb2O5 on Ti for use as an electrode in electrolysis, can anyone access this? This sounds like what we want
to do, but what precursors etc do we need?
http://linkinghub.elsevier.com/retrieve/pii/S0167577X0700317...
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I don't think there are any patents on anodic electrodeposition of SnO2 , and few other references of any sort .
Here's another
http://scitation.aip.org/getabs/servlet/GetabsServlet?prog=n...
It is also possible that from a solution of SnCl4 an electroless deposition might proceed by reaction with
NH4NO3 , similar to the method of oxidative soak .
Another possibility is to use a chlorides derived Pytlewski
polymer dried with mild heating then followed by a dip in ammonium nitrate solution , a rinse , another layer of Pytlewski , in sequence repeating ,
with a good baking
to develop the buildup ......at some number of repetitions
found to give a good thick coating , but not so thick as
to develop cracks like a baked mud .
But I still think a very promising experiment would be stannic nitrate , dip and bake . I think the loading of
solids and thickness buildup are going to be quicker there than using chlorides or alcoholates . But the Pytlewski
sequencing could work better than anything else if it can be included in some way as I am thinking it may work ,
perhaps in conjunction with the stannic nitrate in that sequence . The idea I am following is the differing electrostatic charges of these materials
in adjacent layers
should enhance the density and bonding . Pytlewski hasn't been exploited for anode coatings in any of the reported work , but there seems to be no
reason why it
shouldn't work as well or better than other sol-gels , like the alcoholates especially . Dittos for the stannic nitrate .
It shouldn't be hard to make so that's not stopping me .
[Edited on 22-2-2008 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Update: I took a look at the electrodeposition schemes for SnO2 and discovered that these are not anodic electrodepositions at all like one might
suppose , but rather are cathodic depositions within a narrow reaction window
where the metal which would generally be plated out
is subjected to hydrolysis and instead plates as a hydrated oxide . But the general environment for that deposition would likely present the same
sort of reducing condition
relatively speaking , with regards to a catalytic oxide already present on the electrode , as is the case for example when the electrode is immersed
open circuit into a reducing medium
such as a stannous salt . So SnO2 electrodeposition is not promising as a scheme for deposition of SnO2 onto spinel .
That pretty much leaves the electroless sol-gel deposition
from a stannic precursor as the only promising methods ,
using the same stannic salts as have previously been identified as useful . The chlorides , alcoholates , nitrates
or mixtures , at some point of development in their inherent
hydrolysis schemes are the materials which may have usefulness for any dip and bake methods of coating .
I did run across a perhaps useful simplified method for producing stannic hydroxide from ordinary tin salt , stannous chloride . If stannous chloride
solution is carefully just neutralized , barely to the point of turbidity or beginning precipitation , then addition of H2O2 will simultaneously
hydrolyze and oxidize to produce a precipitate of stannic hydroxide upon heating. It is unknown for certain but believed that this would probably be
the alpha stannic hydroxide . I do not have the preparation details , only a brief reference on page 6 of the attached file .
Also I found some old reference material concerning the preparation and nature of a 70/30 stannic nitrate / stannous nitrate solution , and posted the
information and article in the
antimony and tin nitrate thread
http://www.sciencemadness.org/talk/viewthread.php?goto=lastp...
The easily accessible references that are pertinent to this endeavor have been pretty much gotten at this juncture .
There's bound to be obscure references that were missed .
But I have found pretty much what I can find online to the point it is probably left to experiments to reveal more . So I am going to take a break
and then catch up some backlogged other work before I can get back to this .
[Edited on 24-2-2008 by Rosco Bodine]
Attachment: Pages from 265 Analytical_Chemistry.pdf (299kB)
This file has been downloaded 1654 times
|
|
|
FrankRizzo
Hazard to Others
  
Posts: 204
Registered: 9-2-2004
Member Is Offline
Mood: No Mood
|
|
Here ya go:
Attachment: Preparation and properties of Ti-SnO2–Sb2O5 electrodes by electrodeposition.pdf (571kB)
This file has been downloaded 2557 times
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
I'm planning on messing with those two oxidative soak anodes later today, they have a nice white coat of SnO2 (unbaked) on them, I will rinse them in
1.5M HCl as per the paper, and then soak them in .01M Bi 3+ 1.5M HCl solution, rather than the Sb 3+ solution.
Any reasons you guys can come up with why the Bi would not substitute directly for the Sb in this instance? The paper on Bi doping of SnO2 indicates
a 9:1 SnO2:Bi2O3 is preferred.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Theoretically it should work , but it could be an involved study to find the niche conditions where the deposition
proceeds at the proper rate and produces the adherent film desired .
There is likely a narrow range of pH , temperature , and
solution concentration for a hydrolysis to proceed in a way
which deposits an adherent film instead of precipitating in bulk . Basically all these electroless deposition methods have done is to identify that
peculiar set of conditions
where the very gradual precipitation is favorable to forming a growing thickness film . It is something like
producing a heavy morning frost , instead of an overnight
snowfall .....if the analogy makes sense . This process
can occur for the higher valency salts usual hydrolysis mechanisms , which involves no oxidation as does the
oxidative soak , as the hydrolysis is proceeding upon
the soluble salt already at the higher oxidation state .
On the tin nitrate matter , something I have been thinking
about is that the tin nitrate actually has a surplus of oxygen required for the formation of SnO2 on baking ,
and I have wondered about a mixture of stannic nitrate with stannic chloride , as an oxygen balanced mixture ,
if this might be workable as a dip and bake coating .
The same strategy may be applicable to the nitrates and chlorides of other metals of interest .
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | Originally posted by tentacles
I'm planning on messing with those two oxidative soak anodes later today, they have a nice white coat of SnO2 (unbaked) on them, I will rinse them in
1.5M HCl as per the paper, and then soak them in .01M Bi 3+ 1.5M HCl solution, rather than the Sb 3+ solution.
|
@ tentacles
I thought you said in an earlier post that the Co3O4 layer had been stripped off, as happened with mine. Are you having success with the oxidative
soak now, or was your earlier assessment incorrect. I also tried oxidative soak directly on Ti and still nothing seemed to happen, just a bit of
precipitate.
My recent (half-arsed, admittedly) attempt at a doped SnO2 coating, I mentioned in a recent post was a failure. I used H2O2 oxidised SnCl2 and added
some Bi (BTO). I put it on fairly thickly, directly on to etched Ti. In a perchlorate cell, I couldn't get any current until about 5 volts. To get 1.8
amps (50 mA/cm^2) I had to bump the voltage up to 8 or 9 volts. The current was continuously dropping so I guess it was passivating, and I didn't
continue the experiment. I would imagine it had too high a resistance.
I guess these types of coatings vary by several orders of magnitude in resistance, unless the doping is spot on.The coating looked OK, it was like a
thin white glaze on a teacup, but also clear in places. I did 5 dips each dried at 110 oC. and then baked at 470 oC. This was repeated 5 times. The
coating was really hard, and nothing smudged off. There was no sign of any "free" yellow Bi2O3 forming, so it must all have gone into solid solution
with the SnO2.
Hmmmm... I just read the electrodeposition paper a few posts above and THEIR thermally deposited anode only lasted for 10 mins. at 100 mA/cm^2. If
these doped tin oxide coatings are so good, how come they don't last. Why are they being used as precursors for LDO? Even the cobalt spinel lasts much
longer in a (per)chlorate environment especially if 20 coats were applied. Tin oxide is crap, judging from the results of that paper!
[Edited on 24-2-2008 by Xenoid]
[Edited on 24-2-2008 by Xenoid]

|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by tentacles
I'm planning on messing with those two oxidative soak anodes later today, they have a nice white coat of SnO2 (unbaked) on them, I will rinse them in
1.5M HCl as per the paper, and then soak them in .01M Bi 3+ 1.5M HCl solution, rather than the Sb 3+ solution.
Any reasons you guys can come up with why the Bi would not substitute directly for the Sb in this instance? The paper on Bi doping of SnO2 indicates
a 9:1 SnO2:Bi2O3 is preferred. |
Hello,
IMHO the biggest block to all this is wheather or not the dreaded TiO2 will form. It may have already formed or it may form soon after you put the
anode into a test cell.
Perhaps we are a bit hung up on an exact ratio of dopant. The more articles/patents you read the more doping ratios you come up with. The doping
ratios are all over the place.
Getting a minimum resistance for good conduction etc is not a wise way to look at the doping issue, as the bulk resistance of the coats no matter what
the doping ratio, is small enough for our very thin coats to carry current without any voltage drop worth talking about. Stability of the coating at a
particular ratio, I don't know about that either. As I said (with ATO anyways) the 'best' ratio varys all over the place from pat to pat and article
to article. From 25% Sb to 3% (and less).
I don't like the sound of white coats. May be wrong though.
White coats on any of the stuff I did (shake and bake) were always no good.
Paper above is a very bad advertisment for shake and bake. They are a bit scarce on details how they deposited there coating too.
Regarding the Sb Oxide Sn Oxide system (phase diagram) it is not simple. See over in the refs section for a paper on the system. The same or similar
suituation may be for BTO as well.
Dann2
[Edited on 25-2-2008 by dann2]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
@Xenoid , regarding the higher voltage requirement ...
That was why I urged keeping the spinel interface , the
conductivity , the anti-passivation of the Ti is simply unsurpassed by the other materials .
Comparing the example 2 of US3243385 with the old article about preparation of Dyers Stannic Nitrate ,
via cautious slow dissolution of Tin metal in ~30% HNO3
the process done at about room temperature , it appears
likely that the material gotten from the patent is probably more stannic nitrate than stannous nitrate , perhaps
the same 70/30 mixture of the two as is the dyers stannic nitrate .
But I think to remove all doubt about the +IV valency composition , and also to avoid the byproduct ammonium nitrate impurity , the best approach is
to just make the
+IV alpha stannic oxyhydroxide , settle and rinse the
product by decantation , drain it as much as posssible
and then use the still moist precipitate to neutralize
cool HNO3 . I think that this material , perhaps in admixture with Bi(NO3)3 , perhaps along with some of
the higher valency chlorides of tin and bismuth , and
perhaps also with a bit of ferric chloride or ferric nitrate ,
could give a good dip and baked coating compatable with the spinel , applied onto it , or mixed with the cobalt nitrate and or manganese nitrate
precursors .
Hehehe I am not discouraged at all about the stannic nitrate , on the contrary what I have found out makes it seem more plausible . It's not
something that can be bottled and sold as a reagent because of its short shelf life . Simply because it is a reagent which must be freshly prepared
for use has probably been an inconvenience
which has eliminated it from a lot of experimentation ,
but that doesn't mean that it isn't good for the thing we
are attempting to do . Actually there is an assortment of unstable preparations which have value but which require
being freshly prepared for use , and cannot be kept for
later use . IIRC many of the color test and biological test reagents are unstable , limited use or one use materials ,
but have no more convenient equivalent substitutes .
[Edited on 24-2-2008 by Rosco Bodine]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
The 'Higher voltage' thing is misleading IMHO.
(There is no point in having a discussion about it, my post of 16-12-07 here
http://www.sciencemadness.org/talk/viewthread.php?tid=9572&a...
gives my 2 cents worth) Lets the anode makers make up there own mind on the figures.
If you do a calculation of resistance as seen by our current leaving the anode (travelling through the thin layers) the bulk resistance will need to
be in tens/hundreds of kilo ohms for (mega ohms for TiO2, it certainly makes a gigantic difference) a voltage worth talking about to appear accross
the layer.
@Roscoe Where are you getting the information that Spinel is unsurpassed as an anti Ti passivating layer?
Is Cobalt Oxide an Oxidizer, a strong Oxidizer like LD?
I am not saying you are wrong. Perhaps the spinel is the best ever, better than anything before..........but I have me doughts.......
Dann2
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
| Quote: | Originally posted by dann2
Hello,
IMHO the biggest block to all this is wheather or not the dreaded TiO2 will form. It may have already formed or it may form soon after you put the
anode into a test cell. |
Ummm yeah , that's a passivated anode and a dead cell .
| Quote: |
Perhaps we are a bit hung up on an exact ratio of dopant. The more articles/patents you read the more doping ratios you come up with. The doping
ratios are all over the place. |
The ratios are all over the place when the method employed
uses precursors where the dopant material is volatile during baking , for example the chlorides , as an indeterminate portion of the dopant is lost ,
volatilized during the bake .
| Quote: |
Getting a minimum resistance for good conduction etc is not a wise way to look at the doping issue, as the bulk resistance of the coats no matter what
the doping ratio, is small enough for our very thin coats to carry current without any voltage drop worth talking about. Stability of the coating at a
particular ratio, I don't know about that either. As I said (with ATO anyways) the 'best' ratio varys all over the place from pat to pat and article
to article. From 25% Sb to 3% (and less). |
For the outer working coatings , the integrity of the film is
not so important , and the bi-electrode
effect of islands of dopant as a separate phase may even
have added catalytic benefit . But for the oxygen barrier coatings , doping is absolutely *not* allowed to be all over the place , as when the dopant
percentage exceeds saturation , the SnO2 lattice ruptures and you have a loosely
plugged hole there where the separate phase of dopant sits
there like a cinder in a ragged hole of discontinuous ruptured SnO2 layer surrounding that island of separated dopant with jumbled and broken plates
of SnO2 . A frozen lake is what
is desired , rather than a boulder strewn cracked mud flat .
| Quote: |
I don't like the sound of white coats. May be wrong though.
White coats on any of the stuff I did (shake and bake) were always no good.
Paper above is a very bad advertisment for shake and bake. They are a bit scarce on details how they deposited there coating too.
Regarding the Sb Oxide Sn Oxide system (phase diagram) it is not simple. See over in the refs section for a paper on the system. The same or similar
suituation may be for BTO as well.
Dann2
[Edited on 25-2-2008 by dann2] |
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
I agree that if we can get a 'frozen lake' as opposed to 'cracked mud' it will be much better.
But since we are using a valve metal the cracks will be 'looked after' by the valve metal. (TiO2 will form if O gets into the cracks).
If using an attackable substrate then the 'frozen lake' would be ABSOLUTELY NECESSARY, 'craked mud' a disaster.............but we are using Ti.
Is not all our debate coming from the fact that SnCl4:5H20 is not OTC, therefor we are trying to use SnCl2 or 'homemade' SnCl4 (H2O2 + Stannous) (or
something else that is 'gettable').
If SnCl4 were easily available we would all be quite happy with the 'cracked mud'.....I presume?
Lots and lots of articles etc use the 'cracked mud' as anodes both bare and with overcoats.
I must conceed without a doubt that Sol-Gel, and others have been shown to work for ANODES  . I will have to withdraw the shaking head from way back. . I will have to withdraw the shaking head from way back.
For LD anode on Ti the function of the SnO2 is not to seal the substrate from electrolyte, it is to seal the Ti from the LD so that the LD does not
Oxidize the Ti. (more like saving the anode from itself!!) If it succeeds in doing this in places only that will be OK, so long as there is enough of
SnO2 links to the LD to carry the current. Let the other places on the Ti form a coating of TiO2 (to hell with them) .
Going off topic......
Perhaps it would be more wise to try making SnCl4:5H20 as per the S. C. Whack book (posted by Roscoe) and going the old tried and (successful dare I
say) coldron stirring drones route of shake and bake.
The H202 + Stannous Chloride way does not (alas) seem to work.
Dann2
[Edited on 25-2-2008 by dann2]
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
@ Rosco
I'm getting very confused with all this ... 
For example, in the case of Hubert I put on 10 coats of MnO2. Hubert did not directly die from "passivation", he died because the MnO2 (and the Co3O4)
coating basically physically wore away over a period of 6 weeks or so. In fact when I removed the anode, the lower half still had a black coating,
because it was thicker here due to the "dip solution" running down the hanging anode during pyrolysis.
If I had put a DTO layer between the Co3O4 and the MnO2 the anode would have lasted for 10 minutes longer ....  according to the results in the paper above! according to the results in the paper above!
If I had simply put on 5 dip coats of MnO2 (with drying at 100 oC.) then baking at say 400 oC. and repeated this 10 times ( for a total of 50 coats)
the anode would have lasted for at least 30 weeks as it is a simple physical wear effect. This coating scheme is only slightly more arduous than what
I did initially.
In fact 50 coats of Co3O4 (may be with Ni or Zn) may last just as long (remember my 4 coat anode lasted for a couple of weeks in chlorate).
Basically, I don't see the point of a DTO layer if the "working" coating is going to PHYSICALLY wear away!
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
xenoid: The paper above used electrolysis of H2SO4 to determine anode durability, who knows how that compares to anodic life in a perchlorate cell?
More or less, I just wanted to try this on these presumed failures. If it doesn't work, no big loss. It does not *appear* to be TiO2 - as I said,
there is still residual Co in areas of the substrate, and this white powdery coating evenly covers everything. I will take a picture....
Here's a couple pics of the best example:
http://www.apcforum.net/files/DSCN7225.JPG
http://www.apcforum.net/files/DSCN7224.JPG
[Edited on 24-2-2008 by tentacles]
|
|
|
hashashan
Hazard to Others
  
Posts: 255
Registered: 10-10-2006
Member Is Offline
Mood: No Mood
|
|
Why is it going to wear away? LD doesnt wear away that easily
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
| Quote: | Originally posted by dann2
Hello,
I agree that if we can get a 'frozen lake' as opposed to 'cracked mud' it will be much better.
But since we are using a valve metal the cracks will be 'looked after' by the valve metal. (TiO2 will form if O gets into the cracks).
If using an attackable substrate then the 'frozen lake' would be ABSOLUTELY NECESSARY, 'craked mud' a disaster.............but we are using Ti.
|
It won't matter when nascent oxygen permeation through
a ruptured SnO2 lattice is occuring whether you have cracks along with the open sieve everywhere else , as one problem goes along with the other and
both are fatal to the anode , it's just a race between the two effects to see which passivates the entire substrate first , while they are partners in
crime . The solids content above 8% in a liquid carrier can result in a too thick film for sintering without cracking , no matter what ratio of
dopant is present , but
too much dopant will do the deed all by itself .
| Quote: |
Is not all our debate coming from the fact that SnCl4:5H20 is not OTC, therefor we are trying to use SnCl2 or 'homemade' SnCl4 (H2O2 + Stannous) (or
something else that is 'gettable').
If SnCl4 were easily available we would all be quite happy with the 'cracked mud'.....I presume? |
Actually SnCl4 , or SnCl4-5H2O , or solutions thereof are perfectly OTC for me since I have the equipment to make it .
So the origin for reagents is a non-sequitur . It is like
saying that when you get your Vitamin C from an orange
it is the good stuff , but if it is made in a laboratory as a synthetic , that somehow it must be different . I have the laboratory reagents , and
the base metals , and use whichever is most convenient or sometimes which is most economical . Sometimes I make reagents myself just to be certain
what I have , or to avoid being bent over on something that is overpriced to an unbearble extent by suppliers who seem to believe there is no
alternative source .
| Quote: |
Lots and lots of articles etc use the 'cracked mud' as anodes both bare and with overcoats.
I must conceed without a doubt that Sol-Gel, and others have been shown to work for ANODES  . I will have to withdraw the shaking head from way back. . I will have to withdraw the shaking head from way back.
|
Yeah , you didn't realize it , but when you reflux a chloride
with an alcohol , you are using a sol-gel technique of a sort , just as is the nature of all these hydrolyzable salts inherently
a sol-gel process , even the spray pyrolysis methods ....it just happens quick with that one , whereas these other schemes drag it out longer the
reactions that are occuring .
But why use a method that is imprecise and variable , if
a similar method having better predicatbility is as easy or even easier ?
| Quote: |
For LD anode on Ti the function of the SnO2 is not to seal the substrate from electrolyte, it is to seal the Ti from the LD so that the LD does not
Oxidize the Ti. (more like saving the anode from itself!!) If it succeeds in doing this in places only that will be OK, so long as there is enough of
SnO2 links to the LD to carry the current. Let the other places on the Ti form a coating of TiO2 (to hell with them) . |
The SnO2 is an oxygen barrier for the conductive interface ,
wherever the oxygen may come from . Dopants are used
to do two things , make the SnO2 conductive , and to fill
the lattice with something which will act as a barrier to
migration of atomic oxygen through that SnO2 lattice to
the interface . Bismuth evidently works best , Cobalt and
Iron are about next best , and Antimony is about fourth down the list in effectiveness as that oxygen barrier 
Know you gotta love hearing that 
| Quote: |
Going off topic......
Perhaps it would be more wise to try making SnCl4:5H20 as per the S. C. Whack book (posted by Roscoe) and going the old tried and (successful dare I
say) coldron stirring drones route of shake and bake.
The H202 + Stannous Chloride way does not (alas) seem to work.
Dann2 |
Oh the SnCl4-5H2O works true enough if you get your
alcoholate derivative pH just right , and it would work even better using Co doping than Sb doping , if you simply must
use SnCl4 like there is something holy about it , even though there isn't . SnCl4 is just one of the *two* conveniently soluble stannic salts from
which we may choose , and it's
awkward use and variable results are well described , since it was the most convenient reagent for researchers to use .
But the nitrate is the other soluble reagent which may be useful , as the patents acknowledged , and which from a theoretical standpoint is a
"cleaner" and higher precursor with respect to the reactions through which it must pass in depositing the desired SnO2 . There is nothing illogical
about
trying an alternative or alternatives which may shorten the work of building coatings thickness via chemical reactions
and physical chemistry which is more favorable to the desired end result . Some of the things I have proposed
may seem ridiculous to you , but are never the less supported by theory and also by individual references whose
pertinence I have recognized is knit together towards getting
the alternate scheme to work . The analogy between the
Pytlewski polymer and subsequent coating with colloidal silica
is good basis for my hypothesis that sol-gel SnO2 will very probably likewise adhere , has a high probability of being true .....even though I
challenge you to find that stated in any textbook or patent , I'll put fifty dollars US on it right now . You see it can be a safe bet because
colloidal SnO2
and colloidal SiO2 are remarkably similar in their chemical and physical properties . Therefore it is no far reach to
hypothesize that alternating coats of things which are by nature film formers , and attractive to each other by their
differing electrostatic charges , would stick like hell to each other in adjacent layers , and build thickness quickly of
dense high quality films . What seems to square with theory
and what jumps off the pages in that regard as pertinent
is not something I can pass over as insignificant or irrelevant . It may seem like I have gone off on a tangent ,
because you don't connect the dots I am seeing in these
references I have been sleuthing . And you seem to have some preconceptions about the chemistry involved , or
maybe about chemistry in general .....which causes you
to disregard any justifications I may give about dopants
or whatever other detail .....so beyond that I don't know what else to say .
How about a song from an old accordion player ?
It all started the first time I snatched up a piano
and just squeezed it  Noooo ??? How about Noooo ??? How about
some gospel music ? Okay , time for a diva break 
http://www.youtube.com/watch?v=jWk8TwaqXBI&feature=relat...
@Xenoid , remember the "modifier oxides" which were mentioned being added to the working coatings ?
SnO2 was one of those and serves as a binder and hardener
and toughener for the working coatings .
[Edited on 24-2-2008 by Rosco Bodine]
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | Originally posted by hashashan
Why is it going to wear away? LD doesnt wear away that easily |
Well I'm not referring to lead dioxide, I don't have any experience with it.
I am referring to Co3O4 and MnO2. The MnO2 anode I made, essentially died from the coating being physically and/or chemically worn away from the
anode. The evidence for this was the MnO floating around in the cell and the appearance of the anode when I stopped the cell. The lifetime of the
anode in a chlorate cell (at least) is proportional to the coat thickness. In fact it may be possible to put on a really thick electrolytic coating of
MnO2 over Co3O4 and baking at 400 oC. (as in the Beer - Diamond Shamrock Patent US4444642)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
@Xenoid
Go back and look at US4072586 page 3 column 3 line 56 .
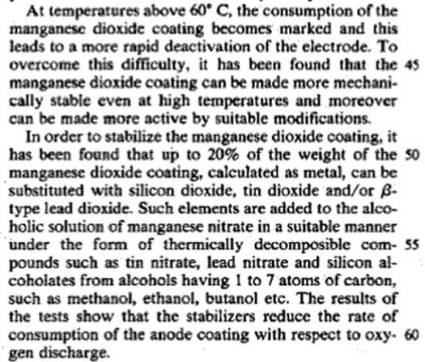
Notice what's the first thermically decomposable compound
listed as an up to 20% addition to the Mn(NO3)2 .
Haven't you wondered why that is the *only* tin compound listed ,
instead of the usual list of assorted tin compounds ?
Since there is only one choice listed , maybe the others
don't work . And for what reason would the one which
doesn't work be the only one example listed of what
does work ? Hmmmm ? 
It looks to me like they are saying something interesting there without elaborating on it .
Also if you look at example 6 , test anodes 4 and 5 which
included some (2%) cobalt doping of the manganese dioxide
had *triple* the endurance of the manganese dioxide alone .
So it is evident that modifying the MnO2 can toughen it significantly .
And the patent makes it clear that the MnO2
alone won't hold up which is precisely why the modifiers
were used and tested . If the MnO2 alone was good enough
then they wouldn't have bothered trying to improve it , as
they successfully were able to do .
http://www.youtube.com/watch?v=eKfDB9Osa88
Essentially the tin nitrate would serve the same purpose
as the silicon ethylate and should bring down the wear rate
to nil for the MnO2 . The 2% Co might be included , but
the Bi would probably be better substituted or possibly in addition with the Co . And all of this over a sealed spinel interface should give you a
bona fide perchlorate anode
baked system . If the chemistry of the coatings is correct
and the film integrity good on the near substrate sealing layers , then having a low wear rate on the working coatings
won't take so many coats , probably a dozen or less from
the spinel interface to completion of the anode , not twenty or thirty .
[Edited on 25-2-2008 by Rosco Bodine]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
@Xenoid
I never seen that patent before. It effectively says that you can coat MMO with Lead Dioxide (or Mn Oxide).
Your pool chlorinator or other MMO's (purchased) as a LD substrate or MnO2 substrate.
Regarding coating wearing away as opposed to the electrolyte 'getting in between' the Ti and the active coating and causing TiO2 to form:
My ATO anode wore away (17 days in a Perchlorate cell). It was 'cracked mud' morphology. Cracked mud is OK.
One final 2 cents about smooth glassy coatings of Tin Oxide (if they are achievable). Your next coat (whatever that may be) may not stick so good to a
glassy smooth coat. It may be that you will be far better off with a craked mud type coat. I would imagine this to be true with electroplated coating
anyways. Baked coats, perhaps not.
If you want a thick coat of Mn Oxide the handiest way is to electroplate it on and then bake. This was done in a patent but I cannot remember which
one at this point in time.
I started to coat a Ti substrate with a coating of Cobalt Oxide on it with Alpha LD (Lead Tartrate bath).
BTW, my Cobalt Oxide coatings are jet black, other peoples seem to be blue?
I posted some Perchlorate cell chemisty stuff over in the reference section if anyone is interested.
Ebonex anyone??????????????????????
Dann2
|
|
|
chloric1
International Hazard
    
Posts: 1147
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
My cobalt oxide also had a bluish hue. When I did nickel over cobalt it became jet black.
Fellow molecular manipulator
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | Originally posted by dann2
Hello,
@Xenoid
I never seen that patent before. It effectively says that you can coat MMO with Lead Dioxide (or Mn Oxide).
Your pool chlorinator or other MMO's (purchased) as a LD substrate or MnO2 substrate.
|
Yeah, I found that one while looking for something else, I just assumed it would have been posted in one of the threads, but I never checked. Yes, you
are correct, plating LDO or even MnO2 on to one of my chlorinator anodes is exactly what I was thinking of doing! When I'll get around to it, I don't
know! The LDO should adhere to the roughish MMO surface and the mesh structure of the anodes quite well!
| Quote: |
If you want a thick coat of Mn Oxide the handiest way is to electroplate it on and then bake. This was done in a patent but I cannot remember which
one at this point in time.
|
'Struth!... dann2 - your memory is going, it's in the above mentioned Patent - US4444642 ... 
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Undoped MnO2 is a catalytic oxygen selective anode ,
which isn't the right material for chlorate or perchlorate .
|
|
|
| Pages:
1
..
6
7
8
9
10
..
13 |