| Pages:
1
..
3
4
5
6 |
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
Would Tungsten do?
It is a 'valve' metal and is OTC as it is used for welding electrodes in TIG welders. It cost about 3 bucks for an electrode that is 3mm my about 6
inches long.
Hot HCl does not etch the stuff. I think you need to attach + power it when imerced in the HCl to get rid of any oxide.
Dann2
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
The point of titanium is that it harmlessly anodizes under perchlorate conditions, no? Whereas any other metal, and graphite, corrodes to hell under
the same stress where plating is not quite complete.
Tim
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
All the valve metals anodize under similar conditions.
Ta, Hf, Zr, W, Ti. Ti is the cheapest is sensible sizes and dimensions.
W as a rod for welding (TIG) is OTC.
The others are hard to get/expensive.
Dann2
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Alternatives to Platinum or graphite anodes which could be investigated
Radiant space heaters have pyroelectric ceramic resistors of lead titanate among
others , collectively known as Perovskites these characteristicaly resist oxidation.
Furnace hot surface ignitors are electric glow plug resistors made of
semiconducting silicon carbide or silicon nitride will run 25 dollars and up.
http://www.alpinehomeair.com/viewcategory.cfm?categoryID=10
http://www.furnacepartsplus.com/Furnace-Parts-Ignitors/c1_11...
Electrically conductive sintered alpha silicon carbide:
It is a dense type of SiC, it has superior resistance to oxidation, corrosion,
wear, and to chemical attacks. The single phase SiC also has high strength,
and good thermal conductivity.
Properties Data
Density (g/cm3) >3.05
Vickers hardness (at 1 Kg) 2400
Flexural strength (MPa)Room temperature800oC1200oC1500oC 395403405400
Young’s modulus (GPa) 390
Compression strength (MPa) 4100
Fracture toughness (MPa) 4.5
Thermal conductivity (W/m oK) 100-120
Thermal expansion (10-6/ oK) 4.0
Specific heat (J/g oK) 0.6-0.7
Electrical resistivity (ohm-cm) 0.5-20
Bend creep @ 1000oC and 200MPa for 1000hrs No detectable creep
The process used to prepare this type of carbide is done by hot pressing,
cold forming, and by electrically conductive sintering. This carbide is wear
and corrosion resistance, and can withstand chemical attacks. General
applications for this type of carbide are grinding media, seal rings, high
power resistors, induction heating susceptor, and as burners and crucibles.
.
|
|
|
chloric1
International Hazard
    
Posts: 1147
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
maybe it will resist attack but it does not mean it will promote the oxidation of chlorate neither. It would be a good excercise to put this in a
chlorate cell with 700 grams per liter just to see what happens.
What might be worth noting is these may be good substrates for PbO2 given they resist oxidation at the substrate/ PbO2 interface.
Fellow molecular manipulator
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
The resistance might be too high.
The resistivity of the stuff is given as 0.5 ohm cm
If you had a piece 1cm square by 10cm long it would have a resistance(end to end) of 5 ohms. I suppose thats managable....or is it.
Can the stuff be had in pieces this size?
Dann2
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Hmmm.....! Not quite sure which thread to post this in anymore. People have been generating too many chlorate / perchlorate / electrode threads
lately. This perchlorate thread seems most appropriate.
Well, I've just finished another perchlorate run with my Palloys platinised titanium electrode. I'll post up the processing of the perchlorate run
separately, as I get around to it. For this latest run I used about 850g of moist recrystallised NaClO3. I couldn't be bothered drying it, I am
guessing there was about, say, 700g of actual NaClO3. I used just about all my remaining chlorate from the bottom of several bags. This would all have
been recrystallised at least twice previously, so we are talking about quite pure (low chloride) chlorate here. The chlorate was dissolved in 1 litre
of boiling water and allowed to cool to about 30 oC. No crystals separated, so it wasn't quite saturated. The volume of this solution was now about
1.4 litres. I put this in my 1.5 litre cell and topped it up with a bit of saturated NaClO3 solution.
The cell was run at 8 Amps (5.5V), but the power supply failed after about 20 hours (see the PSU thread) and I switched to a normal 5V computer SMPS,
this resulted in a current of only about 6.5 Amps. The cell temperature reached a maximum of 48 oC on the 8 Amps, but most of the time was about 40
oC. During the run I didn't notice any drop in current, other than when the temperature decreased overnight.
With this run, and after a little additional reading, but against my better instincts I decided to add a little K dichromate. I have never added any
additives to my chlorate or perchlorate cells before! I added about 1/5 of a teaspoon of powder. Playing it safe this time, the cell was run for about
660 Ah.
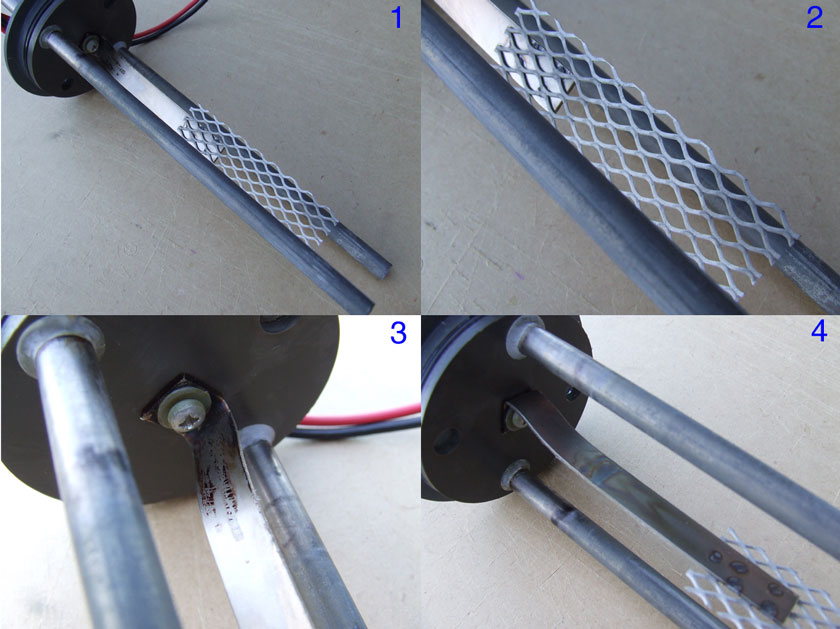
I have attached some images to show the electrode assembly at the end of this run:
Image 1: Overall view, showing two 9.5mm Ti cathodes and inner platinised titanium mesh anode. The mesh is 9cm x 3cm and is spot welded to a Ti strap.
The Ti strap on the original electrode is about 6cm longer, I had to cut it off to fit in my cell. Note discolouration of the Ti cathodes and a bit of
white deposit. There is quite a bit of floc floating around in the cell, I think it is Ti hydroxide from the cathodes.
Image 2. Close up view of the platinised Ti, the variation of grey colour seems to be a photographic effect. To the naked eye, the platinised surface
just looks the same as when I started the run. Perhaps a little mottling here and there. Note the shiny Ti strap.
Image 3. Grey PVC lid and electrode supports. The SS screw and washer are starting to show a little corrosion now. This manifested itself in the form
of brown crud on the headspace part of the Ti strap. This brown crud scratched off easily with a fingernail. The epoxy holding in the Ti cathodes is
now pretty well bleached and is starting to crack. There was a little "salt creep" appearing on the top of the lid during this run. They will have to
be replaced. The PVC is still pretty much unaltered even after 3 runs.
Image 4. The other side of the platinised Ti anode, starting to get a little thin film oxide coating below liquid level. Note discolouration of Ti
cathodes at the liquid level and below.
All in all, I am well pleased with the performance of my platinised Ti anode - SO FAR!
Regards, Xenoid
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Xenoid,
Looks good, yill be coating it with Lead Dioxide yit, I tell ye 
Did you ever find out what is the thickness of Pt on the electrode?
Dann2
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Here's an image of the perchlorate cell in operation, about halfway through the run. It's operating at 5 Volts / 6.5 Amps. Note dark "scummy" build up
due to ?? SS corrosion/dichromate/epoxy degradation.
Regards, Xenoid

|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Here's a closeup of the cell. You can see a little chlorate/perchlorate buildup around the left Ti cathode, where the epoxy is starting to leak.
@Dann2 I mentioned the thickness in the platinum coated titanium thread by Antwain (I think it was there). The Indian site describes the thickness as
2 - 20 microns, but it's not clear if this refers to the variation on one electrode, between electrodes, or that is the range that can be
manufactured. I don't know the thickness of this electrode.
Maybe I got lucky and this was made on a Monday!! 
Regards, Xenoid

|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Very nice Xenoid, I see that you are going the same way as I was, using essentially pure chlorate with a platinum electrode.
My 0,5mm 25cm Pt wire anode carries a maximum current of 4 Ampere and was slightly cheaper than your Pt/Ti anode.
The obvious advantage of the Pt/Ti anode is that it can carry vastly more current per price due to the higher surface and sturdy electrical
connection.
The advantage of a wire anode is that a single run with contaminated chlorate or without dichromate (meaning chlorate reduction at the cathode), e.g.
somewhat erosive conditions, would not destroy it like the Pt/Ti anode could well be due to the very thin Pt coating, but just slightly reduce its
weight and thickness.
Always use dichromate in such a cell! Chlorate reduction at the cathode is a very real problem in both chlorate and perchlorate cells!
I aim on eventually getting a thin square piece of 90% Pt/ 10% Iridium alloy sheet, or some wire out of this alloy. This alloy markedly exceeds pure
platinum in chemical resistance- it is even almost inert to aqua regia, its dissolution only being achievable in reasonable time when it is alloyed
with a large amount of base metal first.
But such a platinized Ti anode would be nice too, and probably more affordable.
A question, does the manufacturer offer a recoating service once the Pt coating has worn off? I have heard of manufacturers offering that kind of
service.
At the moment, I have no source for a Pt/Ti anode though.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|

Here is my platinum plating cell. The urine-colored liquid is worth about $300; hopefully I can get a few anodes out of this. The cathode is
titanium and the anode is graphite. The color is due to PtCl6(2-), with some K+, Na+ and a lot of H+ and Cl- also in solution. The supply voltage is
8V, dropped through 15 ohms, giving 0.44A at a cell voltage of 1.6V. The anode bubbles chlorine gas, while platinum and hydrogen deposit on the
cathode in a black layer. This should become a workable matte silver/metallic coating after burnishing and annealing.
Tim
|
|
|
chloric1
International Hazard
    
Posts: 1147
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
Well Tim I am glad you found a good use for an old muriatic acid bottle.  Say what is the concentration of platinum in your bath? Is it 1%? IIRC somewhere
that gold is electroplated from a 1% auric chloride solution. Say what is the concentration of platinum in your bath? Is it 1%? IIRC somewhere
that gold is electroplated from a 1% auric chloride solution.
Fellow molecular manipulator
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | Originally posted by 12AX7
liquid is worth about $300; hopefully I can get a few anodes out of this..... a workable matte silver/metallic coating after burnishing and
annealing.
|
Yes!... hopefully a few anodes... 
Did you treat the Ti surface in any way?
What will be your burnishing and annealing process? I've managed to coat some etched Ti with my "Hanovia" platinum paint in a baking process, but the
coat appeared very thin, I think it ran for about 12 hours in a perchlorate cell. Unfortunately the baking process needs temperatures around 600 oC to
700 oC. and I believe from one of Dann2's references that there is stress damage to Ti above 500 oC. So don't heat it too much!
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Yeah, about 1%. That was about 5 grams of assorted Pt salts (under the circumstances, I had no way to measure the total), and in almost a gallon
(3.78 l) that's 1.5% or so.
I wet sanded the titanium surface for the most part, and washed with detergent. It wets pretty well ("water should sheet off the surface").
I'm going to torch the metal up to orange heat, 800C or so. Should be enough to get the platinum and titanium to anneal, release hydrogen (thermal
stress vs. hydrogen stress? Figures!) and maybe get some diffusion going. The small scale test I did worked out fine, I have some titanium strips
with a well-adhering platinum plating. Oddly, those came out of the bath with a matte metallic plating, not this black layer. I wonder if the
hydrogen bubbles are causing surface reduction? But, the layer is uniformly black, not in tracks around bubbles or anything. Maybe the pH? I
probably have less acid in this bath than otherwise. I dumped about 200ml 10M HCl, which is less than the ~50ml I put in the much smaller test. pH
is still in the 1 range though.
Tim
|
|
|
chloric1
International Hazard
    
Posts: 1147
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
Wait a minute Tim there is platinum black catalyst. I think this is nano size platinum deposited on platinum. This may be detrimental or helpful
depending on the application. I think catalytic platinum has a lower oxygen overvoltage. Then again you probably could buff it down. Not sure.
Fellow molecular manipulator
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
Yes, the platinized electrode will appear like it's been coated in platinum black. It isn't, it's just the rough nature of the deposit. Upon heating
there is a difference, the black color disappears and the coating evens out, with the Pt diffusing into the Ti. Tim mentioned to me that it is a
semi-gloss metallic appearance on the early trials, after heating.
Proper pretreatment of the anode is imperative.
Pt black is traditionally done with 1% chloroplatinic acid and .05% lead (II) acetate plating bath. I have the exact recipe if anyone wants it. This
K2PtCl6 in HCl is different. Heating a platinum black electrode in a Bunsen flame will still leave a platinum black electrode upon cooling. Pt is
extremely difficult to oxidize, even as a fine powder.
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
It seems to burnish well enough. I imagine it could be useful in this condition as a hydrogen electrode.
Two hours per side is up, I've rinsed, fired (to about 600-800C, unevenly) and am now burnishing part of the finish. The overall color is gray,
somewhat streaked. It seems to be reasonably adherent, not flaking off in any obvious fashion. Some areas darken the finger when brushed, but it's
far from a useless plating.
When I'm done burnishing this area, I'll measure and see how much I put on.
Tim
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
@ Fleaker, 12AX7
Do you know if the plating, annealing process is what is used commercially for platinised Ti electrodes. Or is it a paint based thermal procedure. The
electrode I bought certainly has a metallic matt appearance (looks almost etched).
Looks like a good, simple procedure (albeit expensive) for the amateur, it will be interesting to see how many anodes it is feasible to make from a
given $$$s worth of solution. I await with bated breath.... 
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
On further handling, I noticed that one side was prone to flaking while burnishing. The interface may be sensitive to heat, as I had annealed the
previous burnishing work. Some spots also made contact with my kaowool blanket, which is contaminated with various salts, such as NaCl. I'm not sure
what effect this had, if anything. Annealing to about 900C (orange/yellow color) seems to be not as good. The previously burnished areas seem to be
okay, so I suspect burnishing such a dark coating, possibly after a medium annealing, is the best way to go -- next to a smooth plating, that is. I
tried wirebrushing the surface, which certainly succeeded in eroding the delaminated layer.
I set up a perchlorate cell, consisting of this platinized anode, my stainless steel cathode / cell, about 2 kg of NaClO3, 5 g K2Cr2O7 and enough
water to fill the cell and dissolve the chlorate (some heat was used, to compensate for the cooling effect of dissolving NaClO3). On applying
current, a strong off-chlorine odor was noted (residual Cl- oxidizing, or ClO2?). The anode certainly conducts as it should. We'll see how much
perchlorate forms, and on a longer scale, how long this anode holds up.
Tim
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
| Quote: | Originally posted by Xenoid
@ Fleaker, 12AX7
Do you know if the plating, annealing process is what is used commercially for platinised Ti electrodes. Or is it a paint based thermal procedure. The
electrode I bought certainly has a metallic matt appearance (looks almost etched).
Looks like a good, simple procedure (albeit expensive) for the amateur, it will be interesting to see how many anodes it is feasible to make from a
given $$$s worth of solution. I await with bated breath.... 
|
I'm also impatient. I want to see some damn perchlorate pictures tomorrow ! :-) I think he has very good chances of making perchlorate, he's done it
before with the small Ti and gram of K2PtCl6 I sent him about 4 months ago. Why should he not be able to replicate it now?
As for me, I've plated Pt from perchloric acid solutions and hydrochloric acid solutions both with the same results. You get a matte dark finish,
upon heating and a little rubbing, you can get a dull shine to them. Qualitatively I know it's not titanium that I'm polishing because when I heat it
(about 500C), I do not see the characteristic colourful oxidation bands. Instead, the dark, powdery appearance becomes matte in finish, and just
glows. There is no oxidation, proof positive that something is coating the anode. Pt doesn't oxidize in air at any temperature, so I'm assuming that
is what it is.
In both situations, I prepped my anode-to-be by hot concentrated HCl, rinse, then let it sit in warm 50% perchloric acid, and took it straight to the
cell. I didn't sand it, just gave it acid dip as pretreatment.
I have a feeling that hydrazine sulfate may be quite convenient for a mirror finish (good cosmetically, but it's my hope that it will be resilient). I
still feel strongly that you need more than mechanical pretreatment of the surface for good plating. That is why I sent Tim some ammonium bifluoride.
I'd have to dig up the references, but I've seen pretreatment protocols in the literature about using hot, 70C NaOH, followed by rinse with dH2O,
followed by warm conc. HCl, rinse, then acidified ammonium bifluoride etch, and then a final rinse and into the plating bath. All of this AFTER
sanding. They claimed jewelery grade finishes of varying thicknesses. I need to go back to scifinder scholar and request those journal articles and
patents.
I really am thinking it's how the pretreatment is carried out that has the most effect on the durability and life of the anode. If it's done right,
the Pt coat should last for years. Xenoid's Pt anode that he purchased is slightly reminiscent of what I've made, but mine polished up some to a dull
gloss.
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
It's been, oh, about three hours, and the cell is still bubbling away. It seems to be running quite clean right now; the residual graphite (yeah,
crude NaClO3) doesn't even seem to be around, I wonder if it settled or oxidized(!). No strong odor from this thing, just vapor coming up around the
plastic wadding.
How long will that need, anyway? 2 kg is about 19 mol, and needs 2 e- per, or 53.6 Ah / mol * 19 mol = 1018 Ah. At 30A (seems to be running pretty
consistently at 30A so far) that's 34 hours, or about a day and a half, minus losses. About tuesday morning I should have several kilograms of
perchlorate.
It occurs to me that this really is pretty simple, and yet it's a big accomplishment as SMDB goes. And the key is just platinum. We really are
cheapasses, I guess. 
Then again, if MnO2 and Co3O4 and stuff work out, hell, those are even cheaper!
Tim
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | Originally posted by 12AX7
.... this really is pretty simple, and yet it's a big accomplishment as SMDB goes. |
Humble as usual..... 
30 amps...! How big is this thing, what current density is that... 
Spurred on by Tim & Fleaker's apparent success at platinising Ti, I decided to have another go at using my "Hanovia" Pt resin paint. I've used
this twice previousy on Ti but it passivated after several hours. I've always been worried about using too high a temperature.
I coated a strip of sanded and etched Ti with 3 layers of paint, hot air drying between coats. The resin was then burned off (spectacular flame
effects) using a gas torch and heated to red heat and slowly cooled. This produced the strip shown below, the "rougher" areas had tiny specks of
poorly adherent Pt in them, the smoother areas looked quite promising, as though some diffusion had occurred. When put in a perchlorate cell, bubbles
evolved from all surfaces, but in constant current mode the voltage was slowly rising and it was obviously passivating. I shut it down after an hour!
I don't know why this resin doesn't work better on Ti, as a similar Engelhard Pt/Ir resin was used extensively in the '60's, until it was found that
Pt/IrO2 worked much better and MMO electrodes were developed.
[Edited on 31-12-2007 by Xenoid]

|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Maybe needs a reducing atmosphere?
The electrode is 2" wide by about 8" tall along the plated area, for a surface of about 32 in^2 and current density of 1 A/in^2 or 255 mA/cm^2. I
could give it a lot more!
Tim
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | Originally posted by 12AX7
Maybe needs a reducing atmosphere?
The electrode is 2" wide by about 8" tall along the plated area, for a surface of about 32 in^2 and current density of 1 A/in^2 or 255 mA/cm^2. I
could give it a lot more!
Tim |
The resin itself is supposed to create a reducing environment, it burns off with a very smoky flame! It always seems as though most of the Pt ends up
as little flakes in the residual burning resin. May be I torched it too fast. The earlier versions which were heated slowly and to a lower
temperature, actually had shiny Pt areas, but still had loose flakes.
OK.. ! That's quite BIG, no messing about then!
Oh.. well, back to the pottery chemicals!
|
|
|
| Pages:
1
..
3
4
5
6 |