Swany
Hazard to Others
  
Posts: 188
Registered: 11-4-2005
Location: My happy place...
Member Is Offline
Mood: Sanguine
|
|
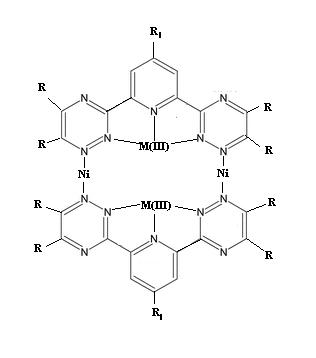
2,6-bis-(1,2,4-triazinyl) pyridines to coordinate multiple metal-centers at once
In the past I have been working with modified bipy to form macrocyclic rings with several coordination centers. However, I need something novel to
try. Since I have been toying with multiple metal center chelations using hefty organic ligands (pretty charge complexes and behavior).
I plan on synthesising 2,6-bis-(1,2,4-triazinyl) pyridine derivs to be soluable in various solvent systems, then I plan to coordinate each with a
trivalent metal using the three central nitrogens, and from there I am wondering if it is possible to form the complex I have attached by somehow
coordinating Ni or Co (II) using the 1-position nitrogens on the triazinyl rings. Literature exists on the M(III) complexes, but I didn't have time to
find anything similar to what I am proposing before my journal access expired (university summer program).
Problems I see are no chirality to cause the dimer to form as opposed to something like a polymer, or steric effects just disallowing it altogeather.
Alternatly, specific R groups could be used to make subunits to be coupled, but the above problem would again exsist, I think.
So in closing:
1) Is there anyway that my attached complex could form, or something like it?
2) If possible, what sort of R-fuctionalization would allow for dimer coupling, and not polymerization?
Some of this is really over my head, but any help/critisism will be greatly appreciated. I hope my post made *some* sense... Thanks!
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
That structural drawing shows 10 N atoms with 4 covalent bonds, as quaternary ammonium salt nitrogens - they would have to each carry a positive
charge, which would be in addition to those carried by the metal cations. The electrostatic repulsion would make such a compound extremely hard to
synthesize, by repeated additions of protons or metal cations or carbonium cations to the unused pairs of electrons on trivalent nitrogens (or
additions of radicals after loss of an electron by each of those N atoms). And what 10 anions do you contemplate having for charge neutrality,
assuming that the Ni present is as covalently bonded Ni(II) and the other metal as covalent M(III).
|
|
|
Swany
Hazard to Others
  
Posts: 188
Registered: 11-4-2005
Location: My happy place...
Member Is Offline
Mood: Sanguine
|
|
I used chloride as my bipy counterion. My ultimate goal is to create something with interesting MLCT chemistry, and I was hoping that this
intermediate species would form, say with the M(III) coordinated and then in the presense of NiCl2 and UV. Alternately, what if it formed a complex
with something very electronegative in the middle of it to sort of stablize it?
However, indeed, I belive it would indeed be stupidly hard to isolate. I am not familiar with trying to make dimers where the other option is
polymerization, say I did a modified Sonogashira reaction using the R groups closest to the Ni atoms, polymerization would be likely. I have heard of
very dilute concentrations being used to prepare the desired product, but... Any ideas? In the end, I want a macrocycle with multiple metal centers
that doesn't nessiserily have to be entirely stable, but maybe only forms in situo.
I really need to read up on coordination chemistry.
|
|
|
|