Per
Hazard to Others
  
Posts: 134
Registered: 26-1-2007
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Perylene production
I read that it should be possible to prepare perylene from napthaline and aluminium chloride but have no more information about that synthesis.
I need it for the peroxodichemoluninescence because of it´s big pi-elektron system. Buing it would be very expensive, 1g/29€.
So I determined to prepare it at myself, but I´m sure that there´s a reason for the price it cost´s commercially.
Maybe a mix of several different organic stuff would be formed.
Because of the fact that the educt napthaline and the product perylene are solid and have a relatively high melting point and sublimates easy, I
suggest that the solvent Toluene should help because napthaline and perylene are soluble in it and it´s not to volatile.
But that´s just speculation.
So does anybody have an instruction for the perylene synthesis?
Or even further suggestions, experiences?
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
You could cook anhydrous AlCl3 in molten naphthalene for a while and then try and isolate perylene from the tars that form. If you use toluene as a
solvent you will likely make a lot of anthracene and toluene/naphthalene adducts. Don't expect a good yield. Think of all the ways two or more
naphthalene molecules could be joined through 1 or 2 carbon linkages.
Google "friedel-crafts naphthalene"
I saw chlorinated solvents used as a reaction medium, also an ethylamine chloroaluminate solvent system. The adducts may not be fully aromatic and
might need further treatment.
[Edited on 9-3-2007 by Eclectic]
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
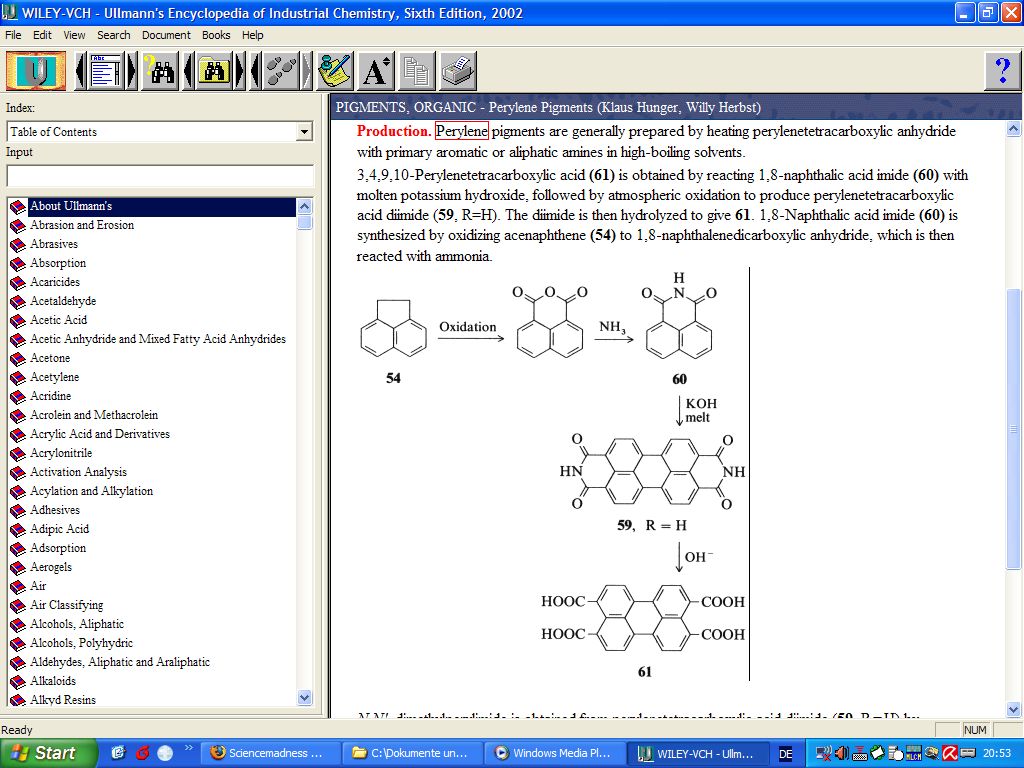
Attached is a screenshot from Ullmann that shows how perylenetetracarboxylic acid is made from acenaphthene.
Perylenetetracarboxylic acid (as itself, or as its anhydride) is an important intermediate for organic pigments and dyes and is mass produced
industrially. You should be able to buy it at a substantially lower price than perylene.
Then you'd have to decarboxylate it somehow.
|
|
|
Per
Hazard to Others
  
Posts: 134
Registered: 26-1-2007
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Thanks,
nox I have instructions for perylenetetracarboxylic acid from acenaphthene, problem ist that I only have napthalene already and just need perylene
and not the tatra-carboxylic acid, which is by the way also a very interesting compound especially for creating more perylene dyes with different
chemoliminescence colours.
The yield shouldn´t be the problem, even 10% would be acceptable because I needn´t huge amount´s of the perylene. Aluminium chloride isn´t a
problem, I got it.
Isn´t it possible to choose the reaction conditions in a way that perylene is preferred formed? When Toluene react´s with napthalene may
chloroforme does work?
There´s another reason why I would avoid working with molten napthalene, it sublimates even at room temperature.
Just hope then that 61°C are enough.
|
|
|