indigofuzzy
Hazard to Others
  
Posts: 145
Registered: 1-10-2006
Location: DarkCity, Bay of Rainbows, Moon
Member Is Offline
Mood: Distilled
|
|
Yellow Percipitate from Sodium Chloride Electrolysis
I've been trying to electrolyze Sodium Chloride to make Sodium Hydroxide. Why? 1. I thought I'd enjoy the challenege  2. Making Sodium Hydroxide just might be easier than buying it. 2. Making Sodium Hydroxide just might be easier than buying it.
Anyway, It probably was a mistake to use copper electrodes, but I was using what was conveniently available in the moment. Aluminum went badly - the
aluminum corroded, fell apart, and left a white precipitate which is most likely aluminum hydroxide.
The copper on the other hand, has been causing a yellow precipitate, and *lots* of it.
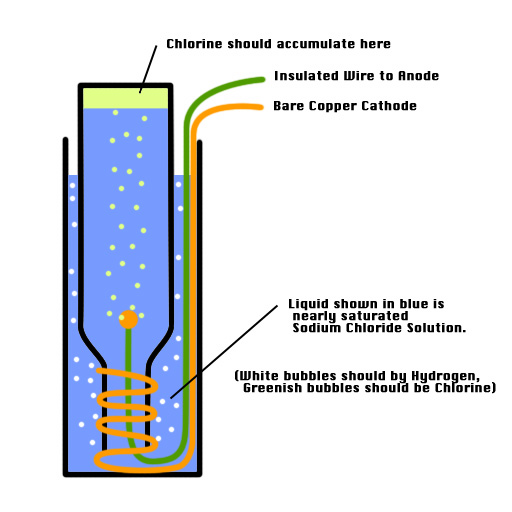
My setup is shown in the attached JPEG.
What strikes me as even more odd, is that when trying to pour off some of the solution from the lower container, the solution in the upper glass
bottle rapidly gets darker yellow. (Orange-yellow, literally the same color as stale orange juice.)
[Edited on 7.19.2007 by indigofuzzy]
|
|
|
chromium
Hazard to Others
  
Posts: 284
Registered: 27-6-2005
Member Is Offline
Mood: reactive
|
|
When electrolysing sodium chloride solution with metal anode result is that solution turns fastly a bit alkaline and after that all metal ions which
go to sultion precipitate as insoluble hydroxides. Only some very inert metals, such as platinum do not decompose rapidly in this kind of
electrolysis.
Without separating anode and cathode areas you will not get NaOH anyway becuse most of chlorine reacts with NaOH in solution making (depending on
temperature and other parametes) sodium hypochlorite or chlorate.
Electrolysis in NaCl solution is widely used to etch metals. You cover your anode with dye or wax or some other inert and isolating layer leaving
blank those areas that must be etched. Process is fast, easy and no dangerous chemicals needed.
When all think alike, then no one is thinking. - Walter Lippmann
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Oh, and the copper ions dissolve from the anode as a Cu(I) chloro complex, which works because chloride is really strong (you would instead get a
flocculent blue precipitate if it were dilute Cl-). On reaching the slightly basic catholyte, Cu2O is formed as a colloidial dispersion, giving a
yellow to orange color. Larger crystals of Cu2O are orange to brick red.
Tim
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
The yellow colour in Sodium Chloride electrolysis has always been a bit of a mystery. Sometimes caused by Iron etc etc.
The following may or may not throw some light on the some possible explanations.
http://www.geocities.com/CapeCanaveral/Campus/5361/chlorate/...
Dann2
|
|
|
chloric1
International Hazard
    
Posts: 1142
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
I made this same mistake 20 years ago. I wanted to turn potassium chloride into the perchlorate. The only information I had at that time was"
potassium perchlorate is produced by continuous electrolysis"in what I think was in Linus Paulings work. Just goes to show what is wrong with the
modern approach to teaching chemistry. Later reading some other text I saw that strong alkali chloride solutions when electrolysed between copper
electrodes, yellow cuprous oxide is deposited. But just imagine a teenager's delight on finding a slightly soluble deposit after an electrolysis
experiment.
Carbon rods. Get carbon gouging rods from welding suppliers. You can make more cuprous oxide stripping the copper coating in sodium chloride
electrolyte>
Fellow molecular manipulator
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Has anyone considered the NaCl source? What if the salt is "iodized?" Could that lead to a precip of NaI? I have not looked up solubility product.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Parts per million.
As a matter of fact, pouring bleach over the stuff results in a brownish yellow color, due to the very small amount of free iodine produced.
You could recover a few grams of iodine if you thoroughly gassed a couple tons of iodized salt with moist, warm chlorine.
Tim
|
|
|
Antwain
Hazard to Others
  
Posts: 252
Registered: 21-7-2007
Location: Australia
Member Is Offline
Mood: Supersaturated
|
|
Yep, its not iodine.
Also, you may have made some bleach in there (maybe) which may explain the slight yellow colour of the solution, depending on how much chlorine you
were actually able to liberate. I agree that Cu2O is probably the more likely culprit.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Duh! Cl2 of course. He's making Cl2 isn't he? Hydrogen at the cathode, chlorine at the anode and NaOH in solution. This is predicated on the
medium being brine not solid dry NaCl.
I edited this because while I see a source of yellowish color in the Cl2 .. I do *not* see a precipiate from my conclusion.
[Edited on 22-10-2007 by chemrox]
|
|
|
Ioxoi
Harmless

Posts: 20
Registered: 24-9-2007
Location: Upstate NY
Member Is Offline
Mood: Pensieve
|
|
I might be in the minority when I say this, but it might actually be CuOH, i.e. cuprous hydroxide.
A while back I was looking at various books and was surprised by the seemingly complete lack of info about copper (I) compounds and their properties,
other than that they were unstable. So I made a fair amount of CuCl (I can't quite remember how; I used copper metal and HCl and copper sulfate in a
reduction reaction), played with it, and learned quite a bit about cuprous compounds. My cuprous chloride was white and insoluble.
Then I reacted the CuCl powder with NaOH sol'n and the result was an insoluble vivid amber substance, which I assumed was CuOH. I might be wrong-
maybe I made something totally different. Anyways, it just might be that the copper metal in your cell is being partially oxidized to Cu(I) and
reacting with generated OH- to form CuOH. The CuOH would then precipitate out in fair quantities.
BTW, after making the yellow CuOH powder and rinsing it to remove excess NaOH, I reacted it with H2SO4 and it changed color, presumably making Cu2SO4.
Can you guess the color of this cuprous compound? Purple! Yes, the substance is water insoluble and forms as fine dark purple crystals. The colors of
copper (I) compounds are odd compared to the typical green and blue of copper(II).
The only problem with cuprous ions is that they air oxidise, for example dissolving CuCl in ammonia yields cuprous chloride diamine solution, but it
quickly air oxidises to form the deep blue cupric amine complex.
Chuck Norris does not uphold laws. He is the law.
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
| Quote: | | BTW, after making the yellow CuOH powder and rinsing it to remove excess NaOH, I reacted it with H2SO4 and it changed color, presumably making Cu2SO4.
Can you guess the color of this cuprous compound? Purple! Yes, the substance is water insoluble and forms as fine dark purple crystals. The colors of
copper (I) compounds are odd compared to the typical green and blue of copper(II). |
You don't get Cu2SO4. Copper(I) ions are not stable in aqueous solution, when no suitable coordinating ligand is present. If you add Cu2O (or CuOH,
but this can better be written as Cu2O.nH2O, as CuOH most likely is non-existent) to dilute H2SO4, then the Cu(+) ions disproportionate:
2Cu(+) ---> Cu(2+) + Cu
The purple color you observe is due to a very intimate mix of red/brown Cu and blue hydrated Cu(2+).
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
| Quote: | Originally posted by 12AX7
Oh, and the copper ions dissolve from the anode as a Cu(I) chloro complex, which works because chloride is really strong (you would instead get a
flocculent blue precipitate if it were dilute Cl-). On reaching the slightly basic catholyte, Cu2O is formed as a colloidial dispersion, giving a
yellow to orange color. Larger crystals of Cu2O are orange to brick red.
Tim |
Hello Tim,
I have made some days ago some Cu2O by this process.. In first experiment I used a small copper pipe , that after several decantation/wash and then
drying gave me a pleasant brick red product you stated.. Since was a quick electrolysis , the amount obtained wasnt that great and so I wanted to make
more.. So I used a bigger Cu pipe as anode.. (the cathode of both procedures was also copper) after the erosion of the anode , decanting, washing
,etc and today after dried the product was light brown.. Why this happen?
Since the PSU was the same (PC PSU) and I've used different copper anodes (well.. the material was the same, the only thing was how much was put as
anode.. So the only thing changed was current density...) do you thing lower current densities tend to gave a more yellow-brownish stuff?
Another thing, I've reacted some of both Cu2O with dillute sufuric acid from used battery acid.. Then quickly a white substance formed around and
dispersed giving a red and very fine substance (copper) which after some time , also dissolved rendering a clear blue solution with no visible ppt..
What you would think is this white substance that forms?
Thanks
[Edited on 15-4-2008 by Aqua_Fortis_100%]
[Edited on 15-4-2008 by Aqua_Fortis_100%]
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Was there a chloride impurity? CuCl certainly forms from Cu2O and HCl. Uncomplexed Cu2O disproportionates in acid, giving red to brown Cu powder and
Cu(2+) in solution.
In some circumstances (possibly: excessive current density, particulates reaching the cathode, unbalanced pH, etc. -- could be a number of things), a
copper sponge forms at the cathode, a brown powder when broken up. Perhaps some of this material got into your product?
Tim
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
Hello Tim,
Really, a copper sponge formed in both procedures.. I dont know why.. IIRC in your web site the liquor was green ( CuCl4-3) but mine has a pale yellow
color , guess from colloidal Cu2O..
But the unstable white stuff formig on reacting with H2SO4 probably was this that you said.. Chloride impurity trapped inside the oxide giving weird
compound..
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|