Freddyhampt
Harmless

Posts: 10
Registered: 18-9-2017
Member Is Offline
Mood: No Mood
|
|
How to remove salt from putrescine dihydrochloride?
What is a simple method to remove the dihydrochloride from putrescine dihydrochloride? In other words, if we begin with putrescine dihydrochloride in
solid form what steps are involved in conversion to just putrescine?
I have been experimenting with sodium hydroxide and ethanol but my procedure must be wrong because the putrescine seems to stay in salt form. I am a
complete novice however.
A step by step description of any ideas would be greatly appreciated. Thank you in advance.
[Edited on 18-9-2017 by Freddyhampt]
[Edited on 18-9-2017 by Freddyhampt]
|
|
|
Metacelsus
International Hazard
    
Posts: 2544
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Treatment with sodium hydroxide should work fine. When you attempted it before, what procedure did you follow, specifically?
|
|
|
Freddyhampt
Harmless

Posts: 10
Registered: 18-9-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Metacelsus  | | Treatment with sodium hydroxide should work fine. When you attempted it before, what procedure did you follow, specifically? |
Thanks for the reply. I have sincerly tried to find the process online but have had little luck so I hoped random experimenting would work. It did
not.
I dissolved the putrescine dihydrochloride in ethanol, then added sodium hydroxide powder. The NaOH did not disolve, so...
I did the same as above but first put the NaOH in solution. Still nothing. So...
I tried to get the pH of the putrescine/NaOH mixture to 11 or 12 by using an electronic pH meter but had strange results. I added a little NaOH and
the pH went up to 10, so I added more NaOH but the more NaOH I added the pH would not climb to 11 or 12. The meter did not max out, it bounced around
the 9 or 10 range, but never got pinned to a max pH.
Why the pH meter did this I do not understand. Must I use only pH test strips?
I believe I should create a 6M solution of NaOH and it dropwise to the putrescine dihydrochloride, correct? Until it reaches a pH of 11 or 12,
correct? Will this cause the dihydrochloride to separate from the putrescine? But should ethanol be mixed with the NAOH solution before adding to the
putrescine dihydrochloride? Is that 'basified ethanol'? Should that ethanol/NaOH mixture be 11 or 12 pH first before adding to the putrescine
dihydrochloride? Or a different way? I have not measured quanties yet, but do have the capability to do so. If I had more info I will do just that.
Thank you again.
[Edited on 19-9-2017 by Freddyhampt]
|
|
|
Elemental Phosphorus
Hazard to Others
  
Posts: 191
Registered: 11-11-2016
Location: Is everything
Member Is Offline
Mood: No Mood
|
|
Putrescine dihydrochloride should be readily soluble in water, no?
I don't see why ethanol should be necesary, the procedure you say you think you should use (without ethanol) seems fine. Perhaps gentle heat might
help in forming the freebase amine.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
The pH effect you got is simply the trimming/buffer effect.
Putrescine is 1,4-diamino-butane... it display two basic groups per molecules
H2N-CH2-CH2-CH2-CH2-NH2 + HCl <--==> ClH3N-CH2-CH2-CH2-CH2-NH2 (or H2N-CH2-CH2-CH2-CH2-NH2.HCl) (monohydrochloride)
ClH3N-CH2-CH2-CH2-CH2-NH2 + HCl <--==> ClH3N-CH2-CH2-CH2-CH2-NH3Cl (or H2N-CH2-CH2-CH2-CH2-NH2.2 HCl - dihydrochloride)
The buffer effect is expressed when you have a free base and its salt together into solution (or a free acid and its salt together into solution); it
expresses when the concentration ratio salt/base or salt/acid ranks from 1/10 to 10/1; usually the pH is "blocked" arround the value of the pKa (thus
+ or - 1 with regard to the 1/10 (0,1) to 10/1 (10) ratio).
Here NH2-CH2-CH2-CH2-CH2-NH2 is the free base of NH2-CH2-CH2-CH2-CH2-NH2.HCl
NH2-CH2-CH2-CH2-CH2-NH2.HCl is the free base of NH2-CH2-CH2-CH2-CH2-NH2.2 HCl
conversely
NH2-CH2-CH2-CH2-CH2-NH2.2 HCl is the acidic form of NH2-CH2-CH2-CH2-CH2-NH2.HCl
So you must have a trimming effect/buffer effect for both pKa's of the diaminobutane...
==> The second one should be (I don't have the precise value but it must be 3 to 4 pKa units lower than the first one) arround 6 (+/-1 that is pH
5-7);
==> The first one should be arround 10,8 (+/-1 thus pH 11,8-9,8... what you observed)
Note that pH-meters/ metry is usually done into water alone... into a mix of water/ethanol or ethanol alone the measures may provide strange readings.
NaOH is quite soluble into ethanol...
I suspect your procedure calls for addition of saturated NaOH/ethanol solution to a saturated diaminobutane dihydrochloride/ethanol; in principle when
stoechiometric amounts are used, one should get the free base in solution into ethanol and a precipitate of solid NaCl (plus a little water formed
into the neutralization process).
***********
To me it would be best (although stinky... putrescine smell like dead meat/zombie (just like cadaverine (diaminopentane; hence their special
alchemist names):
Do this into an expandable recipient/reactor (NaOH will ruin any glassware upon heating; the glass will be corroded and become opaque/white); the
distillation device must be hermetically closeable (except if you wish to get all dead meat eater arround and smell like the morgue/mortuary.
1°) Add DAB (diaminobutane) dihydrochloride to warm water (about 1g/10 ml)
2°) Add excess saturated NaOH/H2O solution and mix the two (it should warm up a little since it is a neutralization and this produces a lot of heat
tempered a little by the initial water
3°) Distil... only DAB and water will pass over (no need of a multiple step/plate distillator... only one plate (a tube) is sufficient (like an
alchemistry horn / retort)... water, NaOH and NaCl will remain into the initial flask (aside with Na silicate from dissolving the glassware).
Enjoy 
[Edited on 19-9-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Freddyhampt
Harmless

Posts: 10
Registered: 18-9-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Elemental Phosphorus  | Putrescine dihydrochloride should be readily soluble in water, no?
I don't see why ethanol should be necesary, the procedure you say you think you should use (without ethanol) seems fine. Perhaps gentle heat might
help in forming the freebase amine. |
Yes, definintely soluble in water. I will try gentle heat and report my findings, thank you.
[Edited on 19-9-2017 by Freddyhampt]
|
|
|
Freddyhampt
Harmless

Posts: 10
Registered: 18-9-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by PHILOU Zrealone  | The pH effect you got is simply the trimming/buffer effect.
Putrescine is 1,4-diamino-butane... it display two basic groups per molecules
H2N-CH2-CH2-CH2-CH2-NH2 + HCl <--==> ClH3N-CH2-CH2-CH2-CH2-NH2 (or H2N-CH2-CH2-CH2-CH2-NH2.HCl) (monohydrochloride)
ClH3N-CH2-CH2-CH2-CH2-NH2 + HCl <--==> ClH3N-CH2-CH2-CH2-CH2-NH3Cl (or H2N-CH2-CH2-CH2-CH2-NH2.2 HCl - dihydrochloride)
The buffer effect is expressed when you have a free base and its salt together into solution (or a free acid and its salt together into solution); it
expresses when the concentration ratio salt/base or salt/acid ranks from 1/10 to 10/1; usually the pH is "blocked" arround the value of the pKa (thus
+ or - 1 with regard to the 1/10 (0,1) to 10/1 (10) ratio).
Here NH2-CH2-CH2-CH2-CH2-NH2 is the free base of NH2-CH2-CH2-CH2-CH2-NH2.HCl
NH2-CH2-CH2-CH2-CH2-NH2.HCl is the free base of NH2-CH2-CH2-CH2-CH2-NH2.2 HCl
conversely
NH2-CH2-CH2-CH2-CH2-NH2.2 HCl is the acidic form of NH2-CH2-CH2-CH2-CH2-NH2.HCl
So you must have a trimming effect/buffer effect for both pKa's of the diaminobutane...
==> The second one should be (I don't have the precise value but it must be 3 to 4 pKa units lower than the first one) arround 6 (+/-1 that is pH
5-7);
==> The first one should be arround 10,8 (+/-1 thus pH 11,8-9,8... what you observed)
Note that pH-meters/ metry is usually done into water alone... into a mix of water/ethanol or ethanol alone the measures may provide strange readings.
NaOH is quite soluble into ethanol...
I suspect your procedure calls for addition of saturated NaOH/ethanol solution to a saturated diaminobutane dihydrochloride/ethanol; in principle when
stoechiometric amounts are used, one should get the free base in solution into ethanol and a precipitate of solid NaCl (plus a little water formed
into the neutralization process).
***********
To me it would be best (although stinky... putrescine smell like dead meat/zombie (just like cadaverine (diaminopentane; hence their special
alchemist names):
Do this into an expandable recipient/reactor (NaOH will ruin any glassware upon heating; the glass will be corroded and become opaque/white); the
distillation device must be hermetically closeable (except if you wish to get all dead meat eater arround and smell like the morgue/mortuary.
1°) Add DAB (diaminobutane) dihydrochloride to warm water (about 1g/10 ml)
2°) Add excess saturated NaOH/H2O solution and mix the two (it should warm up a little since it is a neutralization and this produces a lot of heat
tempered a little by the initial water
3°) Distil... only DAB and water will pass over (no need of a multiple step/plate distillator... only one plate (a tube) is sufficient (like an
alchemistry horn / retort)... water, NaOH and NaCl will remain into the initial flask (aside with Na silicate from dissolving the glassware).
Enjoy 
[Edited on 19-9-2017 by PHILOU Zrealone] |
Very good, thank you. I will try your suggestions.
The problem with pH hitting a wall was just NaOH and water - no diaminobutane. Is there a method to create the 'excess saturated NaOH solution'? A
specific formula?
I am somewhat familiar with distilation apparatus, but not with a closed or 'hermetically' sealed type. How is this made, or what is it called? I am
most curious how it copes with changing volume of distillation.
EDIT: Is the sealed distillation just as simple as this? I would expect it to develop pressure if the erlenmeyer and all junctions were airtight,
no?
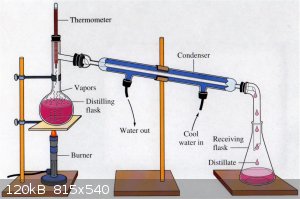
Would not #9 need to lead to some type of expansion chamber capable of expanding to meet the changing volume? If so, what is suitable as an expansion
chamber for this purpose?

[Edited on 19-9-2017 by Freddyhampt]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4416
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Have number nine lead to a trap with some acid in it, to catch any zombie juice trying to escape. And put crushed ice and salt into 16, to keep it
from evaporating.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
@Freddyhampt,
You got it 
It can be hermetical if you have a cold trap as the end recipient; then no pressure build up can occure (assuming you have enough cooling fluid)...
with an efficient cooling the all system can't display any overpressure since any vapour forming is condensing into the cold trap... the pressure into
the system remains thus close to constant.
Exces means what it means...
==> over the stoechiometric amount...
If you know how much DBA dihydrochloride you put into your reactor in grams; then you know the number of moles of DBADHC and because of the 1/2 ratio
for neutralization (1 DBA.2HCl needs 2 NaOH); then you find by conventional stoechiometric calculations how much g of NaOH you would need... any
quantity above this is exces... since NaOH is the cheap reactant... you can work on a 1,5 to 2 fold exces.
[Edited on 19-9-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Freddyhampt
Harmless

Posts: 10
Registered: 18-9-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DraconicAcid  | | Have number nine lead to a trap with some acid in it, to catch any zombie juice trying to escape. And put crushed ice and salt into 16, to keep it
from evaporating. |
Thank you, will do!
|
|
|
Freddyhampt
Harmless

Posts: 10
Registered: 18-9-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by PHILOU Zrealone  | @Freddyhampt,
You got it 
It can be hermetical if you have a cold trap as the end recipient; then no pressure build up can occure (assuming you have enough cooling fluid)...
with an efficient cooling the all system can't display any overpressure since any vapour forming is condensing into the cold trap... the pressure into
the system remains thus close to constant.
Exces means what it means...
==> over the stoechiometric amount...
If you know how much DBA dihydrochloride you put into your reactor in grams; then you know the number of moles of DBADHC and because of the 1/2 ratio
for neutralization (1 DBA.2HCl needs 2 NaOH); then you find by conventional stoechiometric calculations how much g of NaOH you would need... any
quantity above this is exces... since NaOH is the cheap reactant... you can work on a 1,5 to 2 fold exces.
[Edited on 19-9-2017 by PHILOU Zrealone] |
Excellent! Again, thank you. Will report findings.
|
|
|
Freddyhampt
Harmless

Posts: 10
Registered: 18-9-2017
Member Is Offline
Mood: No Mood
|
|
I did not perform distillation because it appears I did not remove the DHC (dihydrochloride). To a solution of DABDHC (diaminobutane dihydrochloride
aka putrescine dihydrochloride) I very slowly added a solution of NaOH. I did this dropwise while stirring and checking pH frequently. At no point
did I get the odor of DAB. I took pH from 7 to 13 using test strips. Because I never acheived the odor I did not attempt distillation.
The NaOH is to liberate the dihydrochloride from the putrescine, correct? Distillation is merely to concentrate the final product and has nothing to
do with the salt removal and conversion to putrescine, correct? My understanding is that the odor should be present once the DHC is liberated from
the DAB. Am I mistaken in any of this?
Please, any suggestions?
[Edited on 27-9-2017 by Freddyhampt]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
The distillation step allows you to leave the salt behind because non volatile.
If your reactants were cold enough... the volatility is less... so should the smell... mild heating should reveal if free DAB base is present or not.
If the pH changed from 7 to 13 then your NaOH is efficient and still active; so you should have smell at ambiant temperature, unless very dilluted
solution or if the DABDHC is not DABDHC but something else?
Edit:
Last option... you are imune to the smell of putrescine and cadaverine... I think it is possible that a genetical modification of the smell receptors
may induce such effect... rare but possible...
==> Ask other people to smell ;o)
[Edited on 27-9-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Freddyhampt
Harmless

Posts: 10
Registered: 18-9-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by PHILOU Zrealone  | The distillation step allows you to leave the salt behind because non volatile.
If your reactants were cold enough... the volatility is less... so should the smell... mild heating should reveal if free DAB base is present or not.
If the pH changed from 7 to 13 then your NaOH is efficient and still active; so you should have smell at ambiant temperature, unless very dilluted
solution or if the DABDHC is not DABDHC but something else?
Edit:
Last option... you are imune to the smell of putrescine and cadaverine... I think it is possible that a genetical modification of the smell receptors
may induce such effect... rare but possible...
==> Ask other people to smell ;o)
[Edited on 27-9-2017 by PHILOU Zrealone] |
Okay thank you. Will try this and report back the results.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Are you sure that you have what you think you have? Where did you get it? What you did is essentially a test for this compound, and if it actually
existed in significant quantities, you'd DEFINITELY be aware of it by now.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
Freddyhampt
Harmless

Posts: 10
Registered: 18-9-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Melgar  |
Are you sure that you have what you think you have? Where did you get it? What you did is essentially a test for this compound, and if it actually
existed in significant quantities, you'd DEFINITELY be aware of it by now. |
A chemical supplier. It has a definite semen smell. It is a yellowish crystalline powder. It is putrescine dihydrochloride, not putrescine.
[Edited on 14-10-2017 by Freddyhampt]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Try adding a small amount to a hot saturated solution of sodium or potassium carbonate, perhaps? There should be be CO2 bubbles visible, and a strong
smell accompanying it. Failing that, try adding it to a saturated solution of NaOH or KOH in hot methanol or ethanol that contains about 25% water by
volume. Test-tube quantities are sufficient. These are just wild guesses, though.
You really should test the melting point and compare it to literature, just to be certain.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
Freddyhampt
Harmless

Posts: 10
Registered: 18-9-2017
Member Is Offline
Mood: No Mood
|
|
Thank you for the ideas.
I will test the melting point with capilary tube in oil. Melt point is specified as just under 300 deg C. Should I use silicone oil, or another bath
oil?
Also, to acheive a saturated solution what is the approx ratio (by mass or volume, mg : ml)?....
Na2CO3 : water
NaOH : ethanol
|
|
|