clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Crystallizing my own piezo pickups (Rochelle salt)?
You might recognize me as one of those infuriating members of this forum who never really gets any chemistry done. I have an excuse -- I share a 500
ft^2 (45 m^2) studio apartment with my girlfriend, and my balcony is very visible from the street so I can't do anything on it. I have essentially no
space to myself.
This, though, I think I might be able to do. I'm trying to understand if I can use crystals of Rochelle salt that I grew myself and attach wires to
them so as to make a guitar pickup. For making RS, I expect to combine equimolar quantities of NaHCO3 and KH(O2CCHOH)2 and recrystallize it until it
gets pretty big. That part probably shouldn't be too hard.
But I'm very fuzzy on the electrical details. Once I have a crystal, I need to encase it in wax, probably immediately, so it doesn't deliquesce. I'm
guessing I want wires on the "top" and "bottom" of the crystal -- defined so the "bottom" is glued to the guitar.
And I need a transistor circuit of some sort in order to normalize the signal at the correct voltage. This I really don't know how to do. Maybe
someone can point me to a book/tutorial?
[Edited on 5-9-2017 by clearly_not_atara]
|
|
|
Sulaiman
International Hazard
    
Posts: 3695
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Online
|
|
Quote: Originally posted by clearly_not_atara  | For making RS, I expect to combine equimolar quantities of NaHCO3 and KH(O2CCHOH)2 and recrystallize it until it gets pretty big. That part probably
shouldn't be too hard.
But I'm very fuzzy on the electrical details. Once I have a crystal, I need to encase it in wax, probably immediately, so it doesn't deliquesce. I'm
guessing I want wires on the "top" and "bottom" of the crystal -- defined so the "bottom" is glued to the guitar.
And I need a transistor circuit of some sort in order to normalize the signal at the correct voltage. This I really don't know how to do. Maybe
someone can point me to a book/tutorial? |
Good luck growing single large Rochelle salt crystals,
unless you have a space lab https://en.wikipedia.org/wiki/Potassium_sodium_tartrate
so you are dealing with a bulk effect, electrical contacts axially aligned with applied stress are common
any circuit designed for an electret microphone element, especially the mosfet type usually included within electret microphones, is suitable.
copied from http://www.scienceprog.com/electret-condenser-microphone-amp...
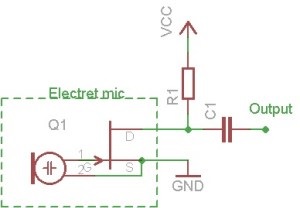
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Rochelle salt is actually one of the easiest large single crystals to grow, provided you have relatively pure material. Otherwise it tends to turn
into crystalline paste or syrup. http://www.sciencemadness.org/talk/viewthread.php?tid=8554
|
|
|
violet sin
International Hazard
    
Posts: 1480
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
https://youtu.be/b--FKHCFjOM
Crystals Go to War 1943 Reeves Sound Laboratories; Piezoelectric Quartz Crystals for Radio.
Does not have direct bearing on the subject, But I just watched this an hour ago. This shows them taking rough quartz to thin and perfect tuned
pieces in finished holders, for radio equipment.
What a pain to get a working finished product. Wow.
1111th post, yay
[Edited on 6-9-2017 by violet sin]
|
|
|
Sulaiman
International Hazard
    
Posts: 3695
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Online
|
|
Possibly useful snippets of information
I came across this line;
"Growth of Rochelle salt crystals must take place at temperature below 40 , above which sodium tartrate is deposited"
in this document Attachment: RochelleSalt.pdf (1.2MB)
This file has been downloaded 669 times
I made a quick solubility chart based on four data points (sorry, I lost the source)
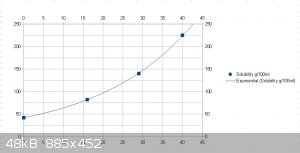
A very steep curve so I guess extremely slow cooling or evaporation is required,
but purification by repeated re-crystalization should be very effective, I think.
Now that I know that large Rochelle Salt crystals can be grown on Earth, I'm going to try it myself.
PLEASE FORGIVE A SLIGHT HIJACK 
I have c400g of "Potassium Sodium Tartrate Tetrahydrate 98%" from a chemicals supplier (no CoA),
This will be my fifst crystal growing attempt so I would like to start with 'pure' Rochelle salt so
my plan is to re-crystalize from water between 40oC and 16oC, 225 and 66 g/100ml
If I start with 225g Rochelle Salt, I could get;
159g after 1 re-crystalization
112g after 2 re-crystalizations
79g after 3 re-crystalizations
etc.
The only method that I have to determine purity is melting point,
and I'm still a m.p. determination noob. (I have capillaries etc)
the m.p. of the tetrahydrate is 74-76 oC
So, I have three questions;
1) Is the general approach correct ?
2) how sensitive is melting point depression to impurities ?
3) how many re-crystalizations is enough ?
I realise that is like asking "how long is a piece of string?" but based on experience,
how many re-crystalizations would you perform to expect to grow large perfect single crystals ?
Based on the CoA from a different supplier, I expect contaminants to be;
SO4, Ca, Mg, NH4, PO4,
======================================
I'm feeble minded ... I just realised that a potentially useful route may be to
Grow a single crystal of Rochelle Salt (RS) from the original stock of impure RS
if not pretty enough then use the crystal grown in the previous crystalization to make a purer crystal,
repeat until happy 
Does that seem like a good approach for a crystal growing noob ?
[Edited on 6-9-2017 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Harristotle
Hazard to Others
  
Posts: 138
Registered: 30-10-2011
Location: Tinkerville
Member Is Offline
Mood: I tink therefore I am
|
|
Copper tape is a great way to attach a pickup.
My recipe involved a two-step purification:
Making a loudspeaker from the stuff in your kitchen.
• Cream of Tartar
• Washing Soda or Sodium Carbonate (which you can get by heating baking soda or sodium bicarbonate in a 275°F oven for an hour)
1. Heat a mixture of about 80 grams cream of tartar in 100 milliliters of water to a boil in a saucepan.
2. Slowly stir in sodium carbonate. The solution will bubble after each addition. Continue adding sodium carbonate until no more bubbles form.
3. Chill this solution in the refrigerator. Crystalline Rochelle salt will form on the bottom of the pan.
4. Remove the Rochelle salt. If you redissolve it in a small amount of clean water, you can use this material to grow single crystals.
Once you have good crystals, taped with copper tape and dipped in wax, connect via an 8ohm:1kohm transformer attached to the crystal. Tape the crystal
to a polystyrene cup. Voila, your own speaker!
This worked really well when I last did it, 4 years ago.
Cheers,
H.
|
|
|
Texium
|
Thread Moved
27-11-2023 at 11:35 |