Photonic
Harmless

Posts: 36
Registered: 29-5-2016
Member Is Offline
Mood: No Mood
|
|
Pressure Swing Adsorption - Designing an Oxygen Concentrator
I am working on a design for a PSA Oxygen Concentrator and need some guidance. In particular where to find rate of adsorption data for 13X type
molecular sieves as opposed to total adsorption.
I have found papers that discuss the total gas adsorbed but they do not discuss the time it was adsorbed over.
For Example:
Pressure (MPa), q/mol*kg^-1
0.105, 0.236
0.325, 0.645
0.695, 1.215
0.805, 1.359
0.99, 1.572
Source: J. Chem. Eng. Data, 2004, 49 (4), pp 1095–1101, 10.1021/je0498917
Many other sources closely resemble this data, and this is one of the few that has high pressure data for N2 over 13X. As some of you may be familiar
with imsmoother's work http://homemadeliquidnitrogen.com/PSA/ he worked with carbon molecular sieves which preferentially adsorb oxygen instead of nitrogen whereas 13X
do the opposite.
I've chosen 13X type sieves over 5A for my design since they perform slightly better, in the order of ~20% more N2 adsorbed per weight quantity. They
also adsorb oxygen far less at 0.4 mmol/gr for 5A vs 0.1 mmol/gr for 13X (Approximate values, derived from graph 3.5 Bar pressure)
Source: Petroleum & Coal 55 (3) 216-225, 2013, "COMPARISON OF TWO PRESSURE SWING ADSORPTION PROCESSES FOR AIR SEPARATION USING ZEOLITE 5A AND
ZEOLITE 13X"
I am aiming for a 15LPM system this gives us 7.5LPM for blowdown, and 7.5LPM for output. That means I need to adsorb 72LPM of N2 (Approximately
92.072g/3.22 mols) to produce 15LPM of O2.
At 1 MPa we can adsorb 1.572 mols/kg of 13X Sieve so if the process were instant we would need 2.05kg of sieves for a 1 minute cycle.
So, I was hoping someone might be able to refer me to some data or equations to help quantify the kinetics of adsorption for Nitrogen/Oxygen on 13X or
sieves in general.
It is also worth noting to those of you doing your own research on PSA for oxygen concentration/air separation the LiX sieves may be better for
separation of oxygen/nitrogen but I have not found a supplier that has them for an affordable price.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
A friend and I were looking to build one of these not too long ago. Here was where we were planning on getting the zeolite:
http://www.ozonesolutions.com/products/Replacement-Parts/Mol...
Communication with various suppliers indicated that units work by pressurizing the zeolite tanks, and then switching tanks when the flow rate dropped
below a set level. You can get flow rate indirectly, by having your air compressor output first through a drying tube, then into a small
pressurizable tank with another line that goes to your zeolite tanks. The first tank can also provide a quick blast of air to your other zeolite tank
when tanks are switched. In other words, considering all the variables you have to take into account, it may be easier to figure out that value
experimentally. You can then store any excess oxygen in a holding tank in order to produce a more even flow. Here's a pretty good introduction to
PSA oxygen concentrators for anyone interested:
http://www.oxygentimes.com/learn/how-oxygen-concentrators-wo...
Oh, also, commercial units usually switch tanks about every 15-20 seconds. You mentioned a 1-minute cycle, although it seems like that may have been
an arbitrary value, and is probably sub-optimal. You probably want to get your air compressor first, then choose your tanks based on how quickly your
air compressor could pressurize them, and your zeolite based on your tank size.
|
|
|
Photonic
Harmless

Posts: 36
Registered: 29-5-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Melgar  | A friend and I were looking to build one of these not too long ago. Here was where we were planning on getting the zeolite:
http://www.ozonesolutions.com/products/Replacement-Parts/Mol...
Communication with various suppliers indicated that units work by pressurizing the zeolite tanks, and then switching tanks when the flow rate dropped
below a set level. You can get flow rate indirectly, by having your air compressor output first through a drying tube, then into a small
pressurizable tank with another line that goes to your zeolite tanks. The first tank can also provide a quick blast of air to your other zeolite tank
when tanks are switched. In other words, considering all the variables you have to take into account, it may be easier to figure out that value
experimentally. You can then store any excess oxygen in a holding tank in order to produce a more even flow. Here's a pretty good introduction to
PSA oxygen concentrators for anyone interested:
http://www.oxygentimes.com/learn/how-oxygen-concentrators-wo...
Oh, also, commercial units usually switch tanks about every 15-20 seconds. You mentioned a 1-minute cycle, although it seems like that may have been
an arbitrary value, and is probably sub-optimal. You probably want to get your air compressor first, then choose your tanks based on how quickly your
air compressor could pressurize them, and your zeolite based on your tank size. |
I highly recommend looking at Delta Adsorbents. While I have no affiliation with them their prices are far cheaper per lb quantity in quantities
greater than 1lb, and they also post specifications on density, shipped moisture content and crush strength.
https://www.deltaadsorbents.com/13x-8x12-molecular-sieve-des...
I have already selected the sieves, the air compressor needed to put out the appropriate flow rates and pressures. All I need to finish the design is
to figure out the adsorption kinetics for 13X Sieves which will determine the cycle rates as well as the bed size and amount of sieves required. I am
not quite sure where to gather this information from, nor am I positive of what term to search for.
[Edited on 10-5-2017 by Photonic]
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I have bought a couple of the cheap 'non-medical' oxygen concentrators via eBay.
I doubt that a diy version would be cheaper, there are many 'bits' required.
Here are some photo's to give an idea, Specified as 2l/min. @ 90% O2, 5l/min. @ 40%
https://www.sciencemadness.org/whisper/viewthread.php?tid=70...
The zeolite stopped working after (I estimate) 4000 to 5000 hours at 2l/min.
The rest of the machine seems as good as new.
Medically, for my mother, they have been fantastic,
as soon as I get around to it, I'll replace the zeolite in the unit pictured.
The thing that surprised me about this machine was the large size of the compressor compared to the zeolite volume.
P.S. the other thing of note is that for a bottom-of-the-line model, there is a lot of air filtering, so it must be very important.
P.P.S. the control electronics
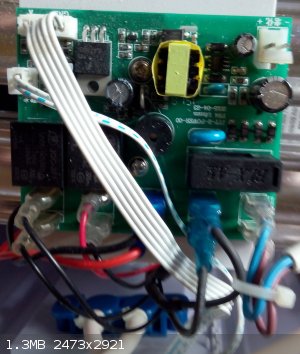
[Edited on 10-5-2017 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Since zeolite slowly becomes less active over time, you should really have your O2 concentrator switch tanks as a response to the zeolite saturating,
rather than try and calculate what it should be doing. That is, compress the zeolite tank until it reaches a preset pressure, then switch
tanks.
@Sulaiman: That looks like it's mostly the circuits for a DC power supply, presumably to actuate the solenoids that control the valves, as well as any
control circuitry. It actually looks pretty simple.
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
the electronics is extremely simple, just a sequential timer,
the most complex part is the motor driven multi-way valve.
(not yet fully investigated, but at least four operations per cycle,
pressurise, de-pressurise, change-over, pressurise......
no other valves required)
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Texium
|
Thread Moved
27-11-2023 at 11:22 |
|