wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Preparation of aluminum nitride
I found this very suprising (to me) and interesting synthesis of AlN in Prepchem.
45 g aluminum powder and 5 g carbon black (lampblack thoroughly) are mixed and placed on a thick iron plate by forming heap and in a safe location.
The magnesium ribbon is inserted into the top of the pile and lighted. The temperature of the burning magnesium is high enough to set fire to the
mixture, but the spot thus ignited is quite likely to cool off before the combustion can get well started. As soon as the ribbon has burned down to
the surface of the pile, the gas flame is pointed over the hot spot until the combustion is thoroughly under way. Although the Bunsen flame alone is
not hot enough to bring the mixture to the kindling point, it prevents the spot heated by the magnesium from cooling rapidly. The combustion is spread
throughout the mixture, and when the combustion is finished the mass is cooled to room temperature. The white aluminum oxide crust from the outside is
removed and the aluminum nitride is preserved in a well-stoppered bottle.
Synthetic inorganic chemistry, by A. A. Blanchard, 153-154, 1936
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Into your process:
You have Al powder and C lamplack mixed into a heap.
You have Mg ribbon and a flamable gas flame to ensure ignition of the pile.
Where is the nitrogen? If it comes from the air, just like the oxygen, how does it reach the core of the reaction media?
Why is the Al2O3 as a crust above the AlN while density of Al2O3 > AlN?
The following german wikipedia link about Aluminiumnitrid states that:
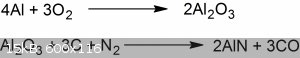
$${\mathrm {2\ Al_{2}O_{3}+9\ C+4\ NH_{3}\longrightarrow 4\ AlN+3\ CH_{4}+6\ CO}}$$
$${\mathrm {Al_{2}O_{3}+3\ C+N_{2}\longrightarrow 2\ AlN+3\ CO}}$$
Happens above 1600°C
While the following above 900°C
$${\mathrm {2\ Al+N_{2}\longrightarrow 2\ AlN}}$$
$${\mathrm {Al_{2}O_{3}+2\ NH_{3}\longrightarrow 2\ AlN+3\ H_{2}O}}$$
[Edited on 23-1-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Dr.Bob
International Hazard
    
Posts: 2734
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Makes no sense to me either, unless the carbon reacts with the oxygen to leave only nitrogen, but that seems like a bad prep to me. Much easier to
heat Al in a nitrogen atmosphere.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Or simply into air leading to a mix with a majority of AlN and a less Al2O3.
Air being composed of approx. 21% mole fraction of O2 and of approx.78% mole fraction of N2 (+1% other gases not taken into account here).
For gases mole fraction is the same as volumic % (1 mole of gas taking 22,41 L at STP).
MM O2 is approx. 32 g/mol
MM N2 is approx. 28 g/mol
AM Al is approx. 27 g/mol
2 Al + 3/2 O2 --> Al2O3
1 mole of Al2O3 is made from 54g of Al and 48g O2.
Al + 1/2 N2 --> AlN
1 mole of AlN is made from 27g Al and 14g N2.
So 100 L of air at STP contains 0,9371 mole of O2 and 3,4806 moles of N2;
or 29,987 g of O2 and 97,456 g of N2.
Those 100 L of air will react with 1,24945 moles of Al for the O2 part (thus 33,735g Al) and with 6,96118 moles of Al for the N2 part (thus 187,952g
Al).
The total mass of Al to completely burn to solid 100 L of air is thus 221,687g...
and it will generate 221,687+ 29,987 + 97, 456 = 349,13g of solid (285,41g AlN (81,75 weight %) and 63,72g Al2O3 (18,25 weight%)).
Assuming density of the solids to be above 3,25g/ccm (density AlN is 3,25 g/ccm and that of Al2O3 is 4,00 g/ccm); this makes at least a solid volume
of 349,13/3,25 = 107,42 ccm.
Since the initial volume was 100 L of gases (or 100 000 ccm) it makes quite a strong reduction of volume (0,10742 % about 1/1000 th).
This explains the strong explo- and then imploding effect of Al powder overfueled detonating mixes into large exploding devices...at first the exces
overheated Al liquid (at the temperature of explosion of the rest of the explosive) reaches the air outside of the explosion fire ball and induces
strong heating and gas expansion...but once the Al has burned the air (both O2 and N2) and it cools down fast and there is some kind of vacuum created
so the winds are sucked back to the explosion center.
[Edited on 23-1-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Yes it is hard to believe the synthesis works.
However while checking the heat of formation of AlN I found a paper titled: Study of aluminium nitride formation by superfine aluminium powder
combustion in air (http://portal.main.tpu.ru:7777/departments/kafedra/tsnm/sotr...)
Though the above synthesis is not identical, It strongly suggests the first method does work though I suspect the AlN is heavy contaminated with
oxide.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
I'm the lucky owner of some such ultrafine Al powder (UAP) with low oxyd layer. It indeed burns into air with two regimes...a first slow and orange,
and then a much brighter white one.
Such Al powder can be light with a simple lighter...no need for a sparkler or for Mg ribbon...the thermite mix Fe2O3/Al or the NH4NO3/Al also...this
by opposition to all the other Al powders I have had.
The profider of that UAP, told me that upon burning into air it produces not only Al2O3 but also AlN
Into you document they expose that Al3C4 may be an intermediary...via the japaneses process.
But here with 5g Carbon for 45g Aluminium...there is a stoechiometry problem:
Based onto your initial post equations you need 1,5 mole C per Al atom; and if you think to Al3C4, it is a little less 1,25 C/ 1 Al.
So you would need for 27g Al at least 15g C (or 18g).
[Edited on 24-1-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Philou: I spotted the less than stoechiometric quantity of carbon. I assumed extra Al was required to keep the temperature of the reaction mass
sufficiently high and the carbon was only an oxygen scavenge in the centre of the reaction mass.
I still wounder why the Al does not simply melt a head of the reaction front. I guess its oxide coating stops that but is sufficently thin not to stop
the reaction with O or N. Perhaps the carbon reduces that oxide coating.
|
|
|