Furch
Hazard to Others
  
Posts: 130
Registered: 8-10-2006
Member Is Offline
Mood: No Mood
|
|
Separation of diastereomers.
Hello.
A friend of mine is having problems separating these two diasteremeric alcohols... (see ISIS-Draw attachement).
So far he's tried:
Flash chromatography with a wide variety of eluents, and flash doesn't really do it for him. It only gives a very slight amount of one of the
diastereomers. He has also tried making the tosylate, mesylate and benzoate... All without any significant results.
L-Tartrate of the pyridine nitrogen and recrystallization, yields the diastereomeric mixture. Since the mixture of the two substances is a "foam", it
oils out rather than crystallize when trying to recrystallize the pure alcohol.
The diastereomer he has been able isolate is a crystalline solid, and the mixture, as mentioned above, is more of a gooey icky substances... More like
glue.
Also, worth mentioning, H-NMR shows that the mixture is pure, i.e. free from contaminations. Also, the diastereomeric ratio is 70:30.
The one thing that hasn't been tried yet is fractional distillation, which is a problem as the synthesis only allows production of a few grams per
batch.
Any thoughts/ideas would be much appreciated!
Sincerely,
- Furch
Attachment: bla.skc (6kB)
This file has been downloaded 930 times
|
|
|
unionised
International Hazard
    
Posts: 5109
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
"a few grams" is about the range where prep HPLC gets used but the kit is expensive.
BTW, I couldn't download the structures.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Separating a couple of grams with HPLC? That's scary!
Furch, were there enough solvents tried for a recrystallization to give up so easily? Though admittedly, it would be a bit difficult to recrystallize
efficiently with a 70:30 ratio.
You should try to form a crystalline salt and try to recrystallize that instead. Why the L-tartrate anyway? You have two diastereoisomers which are
different structures with different physical properties. Therefore, there is no need whatsoever to use enantiomeric acids (these are used to separate
enantiomers by forming diastereoisomeric salts). Try with a sulfate, hydrochloride or whatever else.
Also, why not separating them with normal column chromatography? If trying to do such a large batch as a couple of grams you would need a really big
diameter column (about 15cm!). So you would need to do it a few times in a normal sized one. At least this way you would not lose so much product as
if you would in a recrystallization. If you can't find an eluent that can do a nice separation, then you can still derivatize the mixture (with
acylation for example).
PS: Please be userfriendly next time and provide a picture of your compounds in a legible format and visible in the thread. Like this:
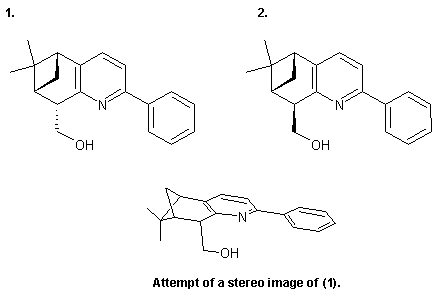
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Drunkguy
Hazard to Others
  
Posts: 172
Registered: 23-12-2005
Member Is Offline
Mood: somewhat pissed.
|
|
15cm diameter for 'such a large batch' as 2g? WTF
Well if the TLC spots of the two diasteromers are so close as to be completely indistinguishable I suggest you find another method. This happened to
me once although it wasnt an issue as it was only by dint of a tosyl containing intermediate.
Firstly you can try recrystallization of the freebase from petrol or Et2O/petrol would be expected to give the major isomer, the minor isomer to be
recovered later.
I've seen a similar scenario for tramadol in a patent (you can search espace for lots of tramadol isomer separation procedures).
Basically the protonated nitrogen of the hydrobromide and the tertiary alcohol are both able to coordinate to the to the bromide, however in practice
this only occurs for one of the isomers and not the other. The patent is not hard to find and I will dig it up for you if it is applicable.
|
|
|