BlackDragon2712
Hazard to Others
  
Posts: 124
Registered: 22-12-2012
Location: Everywhere
Member Is Offline
Mood: Sleepy
|
|
This stuff is eating my lab
So I've just came back to my house for vacations and when I check my lab I found this...

It did something to the walls as well... it has like a yellowish powder sticked on it
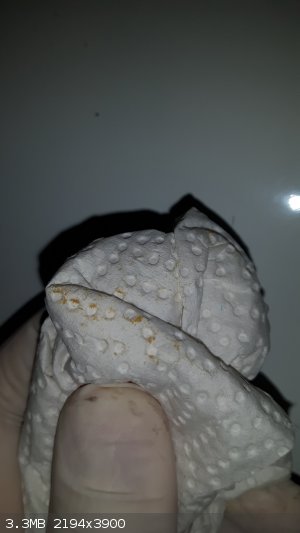
It is also on my gloves...
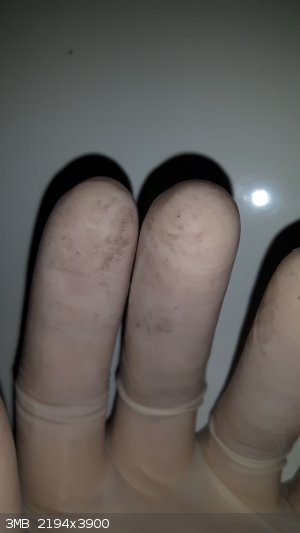
I have no idea of what this could be but here is a list of the stuff I have stored in there:
Mercury metal
Benzoic acid
Sodium acetate
Ammonia
Eugenol
Butanone
Zinc powder
Potassium ferrocyanide
Agar
Dyes
Borax
Potassium permanganate
Phenophthalein
Table salt
Everything is in a separate bottle wrapped in shrink wrap inside a ziploc bag (I did this because my college is in another town so I had to leave my
lab secure enough until I come back for vacations)
I honestly don't know what could be causing this, at first I thought it could be the mercury or the ammonia but I am not sure at all... everything is
cover with this... gray dust and the walls have this yellow stuff... tomorrow I will clean my lab but I want to get rid of the compound that may be
causing this, in the worst case I will get rid of everything I think may be suspicious but I would love to avoid throwing half of my lab away 
Thanks guys in advance!
|
|
|
Texium
Administrator
       
Posts: 4581
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
It looks like the corrosion caused by hydrochloric acid. I know you don't have it listed there, but that's the only thing that I've seen cause that
kind of damage before.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Anything that can:
-produce HCl, HBr or HI or the acid itself (acid halides (mineral or organic), haloamines, haloamides, aqua regia (chloride or bromide + HNO3).
-produce Cl2, Br2 or I2 or the halogen itself even in solution into a solvent.
-produce Hg (Mercury is quite volatile and forms amalgams with metals thus protective Zn layer (galvanisation) is chewed and naked iron is exposed to
air or to the vapour of your chems.
The two first are the best suspects! Since I have experienced this a lot of time with those.
The corrosion may occur even if the source has dissapeared...so maybe you don't have the bottle of HCl or you I2 drum in there anymore ... the
exposure may have been long enough to start the rusting decay...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
It could also be vapours coming from the fridge below the cabinet (assuming you store chems in there...)
|
|
|
BlackDragon2712
Hazard to Others
  
Posts: 124
Registered: 22-12-2012
Location: Everywhere
Member Is Offline
Mood: Sleepy
|
|
mmm... Yes I also have chems in that fridge...
Dicloromethane, toluene, chloroform, THF, and a few experiments I couldn't finish.
I had a bottle of 1 L of 32% HCl stored in a different cabinet but it is in a different location (close though) but a third cabinet that is right
above the one I used to stored that HCl did not suffered corrosion at all...
I used to store iodine metal in that cabinet but before leaving my house I got rid of it and back then that cabinet was not showing any rust (like 1
year ago)
In the worst case scenario... thinking that it may be the mercury vapors... how can I test for mercury in the air? and how should I proceed with the
clean up if it were mercury? I remember reading somewhere that sulfur is used for mercury spills
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
UV light in front of a white paper display shadow fumes if Hg vapour is present.
HCl 3 cabinets away and I2 from last year in the same cabinet may be responsible...corrosion sometimes is inhibited for unknown reason, but once
rusting starts...it goes very fast.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
diddi
National Hazard
   
Posts: 723
Registered: 23-9-2014
Location: Victoria, Australia
Member Is Offline
Mood: Fluorescent
|
|
an interesting idea to check for HCl vapour in the area is to put a small amount of NH3 sln in a beaker nearby. if there is HCl around it will
deposit white powder everywhere
Beginning construction of periodic table display
|
|
|
woelen
Super Administrator
        
Posts: 8014
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I also think that this corrosion is from HCl, HBr or free halogen. I do not belive at all that this strong corrosion can be caused by mercury vapor,
escaping from a closed container.
I had similar problems, not from HCl itself, but from storage of PCl5, POCl3, PCl3 and SOCl2. All of these are ampouled now and I have no corrosion
issues anymore.
I store my HCl in special Schott Duran bottles with GL45 caps having a teflon coated soft silicone liner. I also diluted my 36% HCl to 30%, which
strongly reduces the fuming. The 36% acid even caused trouble when stored in the special Schott Duran bottles, but the 30% acid now is stored for
years already without any issues.
I also have 48% HBr, also stored in a Schott Duran bottle with GL45 cap. No issues at all.
The corrosion in my lab was really severe. In just a few weeks, all copper materials were covered by a thin green layer and making good electrical
contacts was bad. I also stored electronic components and some of these have severely corroded legs, which cannot be soldered anymore. After I
discovered that, I have rigorously removed all possible sources of corrosion.
I also store a few bottles of mercury in my lab, but none of them produces any corrosion issues, not even after years of storage.
|
|
|
j_sum1
Administrator
       
Posts: 6322
Registered: 4-10-2014
Location: At home
Member Is Online
Mood: Most of the ducks are in a row
|
|
Off topic...
woelen, I would love to see photos of your lab. I don't think you have ever posted in the Tour my lab thread.
[/off topic]
|
|
|
BlackDragon2712
Hazard to Others
  
Posts: 124
Registered: 22-12-2012
Location: Everywhere
Member Is Offline
Mood: Sleepy
|
|
So, at the end it seems that the Iodine metal was the problem... checking a few photos I took the last year I found that the corrosion began the last
year and it advanced even when I removed the iodine from that cabinet (the black bottle on the top is the iodine... it is very very annoying since it
escapes everything and stains everything)
I also did what PHILOU Zrealone said but I couldn't detect any mercury vapor so those are good news...
Thanks everyone for the help... I'll be more careful with these stuff
Attachment: phpwVd3Mq (3.9MB)
This file has been downloaded 614 times
|
|
|
woelen
Super Administrator
        
Posts: 8014
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
One way to store iodine (and also chemicals, producing HCl vapors), is to keep the bottle in a small bucket or jar, in which some NaOH is put in a
little dish as well. This exterior bottle must be sealed well, and of course the iodine-bottle must also be sealed. Any iodine vapor, escaping the
iodine-bottle, is absorbed by the NaOH. Once every month or so, you need to replace the NaOH by fresh pellets. A small dish with 5 cm diameter and a
few mm high, filled with 2 to 3 mm of NaOH-pellets/granules is enough. This method is 100% effective. I use this for storing my small remaining
containers of PCl5, POCl3, SOCl2, CH3COCl. The rest (my main stock) of these chemicals is ampouled in glass.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
I think i mentioned this somewhere before recently. I got 5ltr of 36.2% HCl from APCpure in the UK.
The container has an excellent lid on, i have no idea what its made from but has a gasket on it. I must try and take some pics later, normally the
acids are kept in a coldish place (this time of year around 10c).
I forgot a week or so ago while doing the sea shells in HCl, and i left the HCl bottle in a cupboard in my lab, it got to around 22C and when i
remembered and went to get the container the sides had swelled a bit.
There was zero sign of escape of the acid though. Not sure if i would use the company again as they messed me about, but i will be keeping the
container and reusing it!
|
|
|