Velzee
Hazard to Others
  
Posts: 381
Registered: 19-8-2015
Location: New York
Member Is Offline
Mood: Taking it easy
|
|
Question on a compound's atomic structure
This is a rather odd request, but I was wondering if there was a chemical which has a similar structure to the radiation hazard symbol (https://en.wikipedia.org/wiki/Hazard_symbol#/media/File:Radi...). The closest I've found so far is that of chromium(VI)
peroxide(https://en.wikipedia.org/wiki/Chromium(VI)_oxide_peroxide#/media/File:CrO5-2D.png).
This was just a random question that popped into my head as I began to read the Wikipedia page of CrO5. Thank you all in advance!
[Edited on 3/1/2016 by Velzee]
Check out the ScienceMadness Wiki: http://www.sciencemadness.org/smwiki/index.php/Main_Page
"All truth passes through three stages. First, it is ridiculed. Second, it is violently opposed. Third, it is accepted as being self-evident."
—Arthur Schopenhauer
"¡Vivá Cristo Rey!"
—Saint José Sánchez del Río |
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
Weird question , maybe TATP? , maybe TATP?
http://www.123rf.com/photo_19617722_acetone-peroxide-triacet...
[Edited on 1-3-2016 by crystal grower]
|
|
|
Velzee
Hazard to Others
  
Posts: 381
Registered: 19-8-2015
Location: New York
Member Is Offline
Mood: Taking it easy
|
|
Eh, not quite what I'm looking for xP
Check out the ScienceMadness Wiki: http://www.sciencemadness.org/smwiki/index.php/Main_Page
"All truth passes through three stages. First, it is ridiculed. Second, it is violently opposed. Third, it is accepted as being self-evident."
—Arthur Schopenhauer
"¡Vivá Cristo Rey!"
—Saint José Sánchez del Río |
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
What exactly were you looking for, apart from what you have already had ?
|
|
|
Velzee
Hazard to Others
  
Posts: 381
Registered: 19-8-2015
Location: New York
Member Is Offline
Mood: Taking it easy
|
|
A chromium compound with six oxygen atoms attached to it, in the shape of the radiation symbol, or something similar. Again, I'm only basing this off
aesthetics.
P.S. Long time, no see, aga! I missed your presence around here!
Check out the ScienceMadness Wiki: http://www.sciencemadness.org/smwiki/index.php/Main_Page
"All truth passes through three stages. First, it is ridiculed. Second, it is violently opposed. Third, it is accepted as being self-evident."
—Arthur Schopenhauer
"¡Vivá Cristo Rey!"
—Saint José Sánchez del Río |
|
|
j_sum1
Administrator
       
Posts: 6322
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Easy enough to draw. But there is no guarantee that such an arrangement would be stable. And if it was, no guarantee that it would be planar. I can
see the O-O-Cr triangles rotating 90°.
Edit
That is what chemsketch does with it anyway...
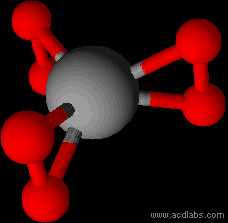
Replacing the Cr with a lanthanide in 6+ oxidation state might fare better.
[Edited on 2-3-2016 by j_sum1]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Huh?
|
|
|
j_sum1
Administrator
       
Posts: 6322
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Yeah. I dunno what I was thinking really. Well, yes I do, but it fails on all counts.
For a start the highest common oxidation state in the lanthanides is Tb(IV). I forgot that.
Might have better success with an actinide but... well, there are more issues.
I was thinking that a higher coordination number afforded by an f block element would give a couple of electron clouds that might push the oxygen back
into a plane. But there are so many problems with that geometry that it failfails.
Let's just call it a spontaneous brain fart and leave it at that.
[Edited on 2-3-2016 by j_sum1]
|
|
|
Mabus
Wiki Master
  
Posts: 238
Registered: 3-11-2013
Member Is Offline
Mood: Energetic
|
|
Maybe some crown ether coordinating a metallic ion, like this one: https://en.wikipedia.org/wiki/Crown_ether#/media/File:18-cro...
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
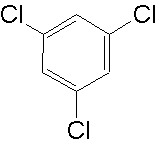
1,3,5-trichlorobenzene ?
I just drew it in chemsketch and pressed ctrl-I to get that name - no idea if it does/can exist
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
https://en.wikipedia.org/wiki/1,3,5-Trichlorobenzene
|
|
|
Velzee
Hazard to Others
  
Posts: 381
Registered: 19-8-2015
Location: New York
Member Is Offline
Mood: Taking it easy
|
|
You see how the bonds of the oxygenated on the CrO5 create triangles similar to the radiation hazard symbol? That's more of what I'm looking for.
Check out the ScienceMadness Wiki: http://www.sciencemadness.org/smwiki/index.php/Main_Page
"All truth passes through three stages. First, it is ridiculed. Second, it is violently opposed. Third, it is accepted as being self-evident."
—Arthur Schopenhauer
"¡Vivá Cristo Rey!"
—Saint José Sánchez del Río |
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Propellane comes to mind.
But also left and right propeller blade you get from complexing hexacoordinating Metalic core with bidentate ligands...like
Ruthenium (III) Tris-PHEHAT...related to figures 43 in the following link http://www.mdpi.com/1420-3049/19/4/5028/htm
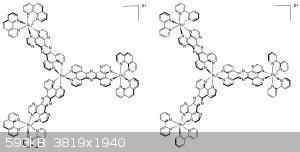
See also Ru tris-bipy
https://en.wikipedia.org/wiki/Tris(bipyridine)ruthenium(II)_chloride
http://www.isu.edu/chem/people/faculty/strodenn/
[Edited on 4-3-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
Guanidinium?
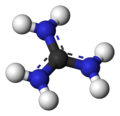
Or it's identically-shaped, neutral, theoretical, boron equivalent triaminoborane, a weird molecule
with negative hyperconjugation (B-N bond length increases in order BH2NH2 => BH(NH2)2 =>
B(NH2)3)
Or the real, isolated B[N(SCF3)2]3
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Surely you meant to say, "molecular structure"
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|